Video-assisted thoracoscopic pneumonectomy: the anterior approach
Video-assisted thoracoscopic surgery (VATS) lobectomy is now a well-accepted way to perform minimally invasive lobectomy and has been shown to be both safe and technically feasible, and garners a number of advantages over conventional surgery, such as reducing length of hospital stay, decreasing blood loss, decreasing pain, improving cosmesis, earlier returning to normal activities, improving tolerance of chemotherapy and so on (1-4). Starting from the 2006 version of the NCCN guidelines, video-assisted thoracoscopic lobectomy has become the standard treatment for early stage non-small cell lung cancer. VATS lobectomy is different from the conventional surgery. Different incisions, instruments and camera positions have also been described in the past two decades. There are no fixed patterns in VATS lobectomy (5-7). The technique of VATS lobectomy described here (Video 1) is currently underwent in the Unit of Cardiothoracic Surgery at the Affiliated Hospital of the Guangdong Medical College.
Clinical summary
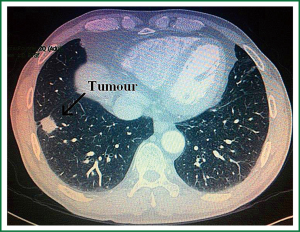
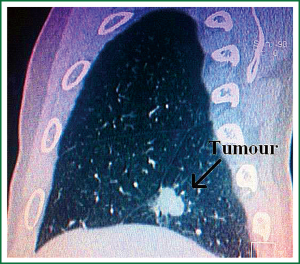
A 57-year-old man with coughing up blood tinged sputum, right chest pain for one month, and both lateral supraclavicular lymph node negative, cardiopulmonary abdominal check no exceptions, 30 years cigarette smoking history was admitted to our hospital. Before surgical resection, the patient underwent preoperative examinations including ECG, abdominal ultrasound, pulmonary function test, thoracic CT scanning, enhanced head MRI and bone scanning. Thoracic CT imaging revealed a 22 mm × 22 mm × 20 mm, T1bN1M0 lesion in the right lower lobe (Figures 1-3), mediastinal lymph node metastases. Lung function was assessed via formal spirometry with an FEV1 of 2.16 (98% predicted), an FVC of 2.73 (97.5% predicted), an FEV1/FVC ratio of 79.1% and a DLCO of 80% (85% predicted).
Selection criteria
Since March 1993, a number of indications have been modified, and—more importantly—the number of contraindications has been reduced. The current indications for VATS lobectomy in our medical centre are:
- tumor <4 cm in size. The authors have removed tumors of up to 6 cm in exceptional cases;
- peripheral location, i.e., >1 cm from the fissure and >3 cm from the lobar carina;
- T1N0 and T2N0 lung cancers. Although this is not a completely exclusive criterion, swollen intrapulmonary or mediastinal lymph nodes do not necessarily contraindicate resection and can be removed completely in many cases;
- open fissures. However, this is not absolutely essential, the authors have dealt with many cases of the incomplete fissure.
Anaesthesia and positioning
Following the double-lumen endotracheal intubation under general anesthesia, the patient was placed in the lateral decubitus position with the arms extended to 90° and the elbows flexed to 90°. To reduce inadvertent injury to the intercostal neurovascular bundles, the table was “broken” or flexed to maximise the intercostal spaces. The surgeon stood facing the patient, with the principal assistant beside him. Two assistants were placed behind the patient. Two operative cameras were located in patient with head on both sides. A 10-mm 30° thoracoscope was used. Our experience tells that the assistant with the 30° lens is more important to the procedure and can have a good or bad influence on the surgeon.
Incisions
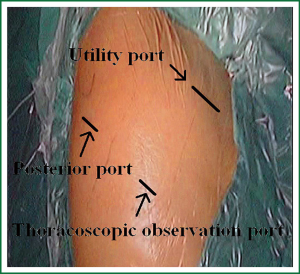
Placement of the incisions is key to the performance of VATS. Proper placement of the incisions creates the best angle for the instruments to perform these procedures. Three access ports are used (Figure 4). Incision 1 is a utility incision through which much of the dissection is performed and a lobe is removed. It is 4 cm long and placed in the fourth intercostal space in the anterior axillary line. The port site incision is made in the upper, middle and lower lobectomy of all patients. The incision through the chest wall is toward the major fissure and the hilus pulmonis so that it automatically directs instruments through this incision. Incision 2 is used for the trocar and thoracoscope. It is placed low in the chest to provide a panoramic view of the chest, which is in the eighth intercostal space in the posterior axillary line. It is angled superiorly so that there is less torque on the intercostal nerve. Incision 3 is 1.5 cm long and placed in the auscultatory triangle, a slightly higher of which can provide slightly better access to help the paratracheal node dissection, or a slightly lower of which can provide a better angle for the stapler to cross the pulmonary vein.
Operative techniques
According to the bronchial and vascular anatomy of different lobes, VATS lobectomy may require an alternate approach. The inferior and superior approaches to the right lower lobectomy performed as VATS are described. In our experience, the order of division does not affect the outcome and therefore safety should be a priority during this part of the operation.
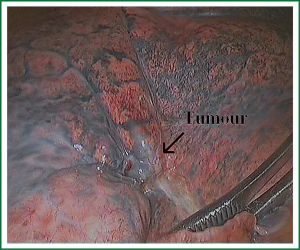
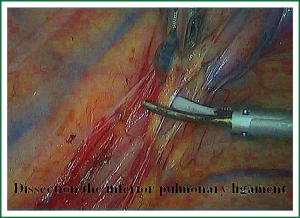
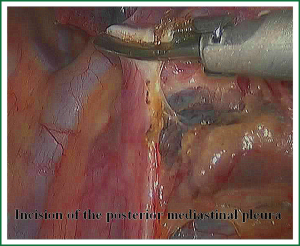
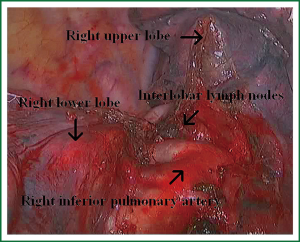
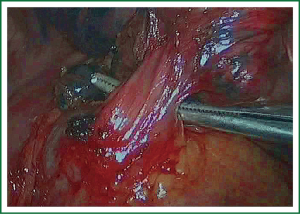
The first step in the procedure is to identify invasion of the chest wall, pleurae and hilar structures, especially to confirm the resectability of the hilar lymph nodes adjacent to pulmonary vessels. Dissection is commenced in the inferior pulmonary ligament, the anterior and posterior mediastinal pleura of the hilar with a combination of blunt and sharp incision sequentially (Figures 5,6). In this process attention must be taken to avoid inadvertent injury to the vagus, phrenic, and tracheal membranous part.
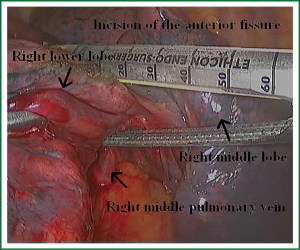
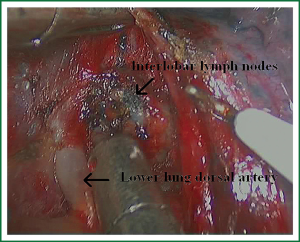
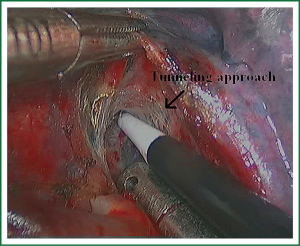
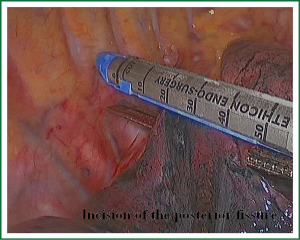
As we know, the differentiation of the fissure has a greater impact on the operative process. In many cases, it needs to change the approach of surgery because of the absence or incomplete fissure. i.e., a unidirectional lung resection is used in these patients (operative order: pulmonary veins, bronchial, pulmonary and fissures). Our experience is the “tunneling” approach. Exposure to the lymph nodes in the inferior, anterior and posterior hilar is to the point in the procedure. The order of division is to expose the station 11 interlobar lymph nodes adjacent to the bronchus between the right lower and middle lobe firstly, and then make the “tunneling” from the bottom up the hilar plane along the surface of the station 11 interlobar lymph nodes, and detach the lung parenchyma by the stapler (Figure 7). The steps of separating the posterior fissure are to expose the station 11 interlobar lymph nodes adjacent to the bronchus between the right upper and lower lobe, and then make the “tunneling” from front to back the hilar plane along the surface of the station 11 interlobar lymph nodes group of lymph nodes, and detach in the same way (Figures 8-11).
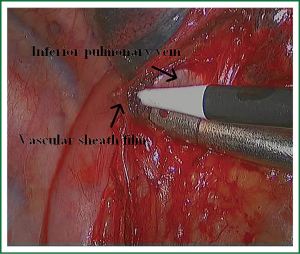
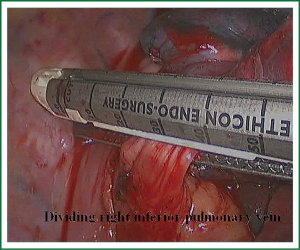
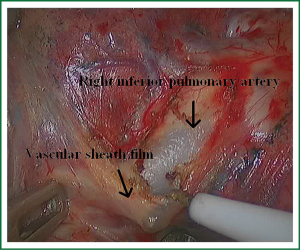
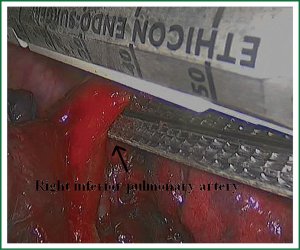
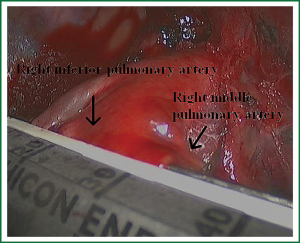
In the case presented here the right inferior pulmonary vein was divided using an endoscopic vascular stapler passed through the utility incision. Avoided injury to the back wall of the vein by directing instrument tips toward the bronchus (superiorly) rather than the vein wall (Figures 12-14). The right inferior pulmonary artery was divided in the same method, and attention must be taken to the right middle pulmonary artery (Figures 15-17). There are some advises to divide the pulmonary vessels in our experience. Firstly, the incision is to the point to the vascular sheath film, and the application of aspirator can create space and avoid injury to the vessels. Secondly, the lymph nodes between the pulmonary vascular branches can increase the difficulties of the operative process. Attention is turned to assess the resectability or the unresectability of the lymph nodes, and change the surgical method in time. Thirdly, the free length of vascular should be as possible as long, in order to avoid the difficulty of suture retractor or tearing vessels. Fourthly, in the case of vascular bleeding, oppress and clamp to cease the bleeding, and then clean the blood, most bleeding can be dealt by sewing and endoscopic titanium-clip. If the bleeding cannot be stopped in VATS, a decision should be made immediately to transform VATS into the conventional surgery.
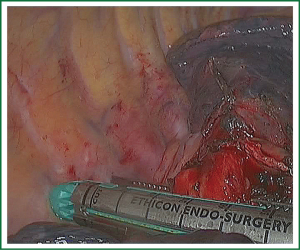
The lung was turned to its anatomic position. Any lymph nodes that obstruct the view of the bronchus should be removed separately or swept so that they could be removed en bloc with the specimen. The right lower lobe bronchus was divided using an endoscopic stapler for thick tissue passed through the utility incision. The middle lobe bronchus must be clearly seen to avoid compromising it with the stapler (Figure 12). The resected lobe was placed in a plastic glove and extracted through the utility incision (Figure 18).
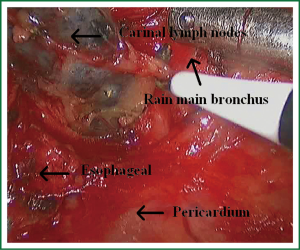
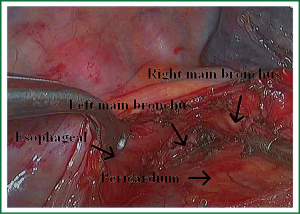
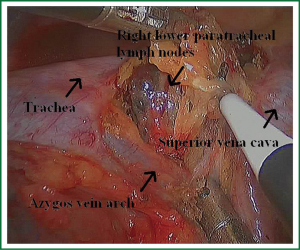
Because of endoscopic zoom effect, lymph node dissection in VATS via thoracotomy has more advantages and can be more conducive to identify the small blood vessels and lymphatic vessels around lymph nodes, which can reduce the complications such as postoperative chylothorax and lymphatic leakage. Attention must be paid to the blood vessels of the carinal lymph nodes, which may come from the esophageal artery branches and bronchial artery branches. The dissection of the carinal lymph nodes would better start from the side of the esophagus to reduce intraoperative bleeding (Figures 19,20). The dissection of the right upper paratracheal (2R) and right lower paratracheal (4R) lymph nodes can firstly open mediastinal pleura of the lower edge of the azygos vein arches, free and pull up the azygos vein arches, remove the right upper paratracheal (2R) and right lower paratracheal (4R) lymph nodes from the bottom up (Figures 21,22).
Post-operative management
The patient underwent a routine postoperative chest tube drainage and chest X-ray. Analgesia, antibiotics and anti-coagulation were administered routinely in accordance with local guidelines.
Comments
Currently, there are many debates on whether VATS lobectomy is more effectively performed via an anterior or posterior approach. Our experience suggests that, given selected appropriate patient and experienced thoracoscopic surgeons, VATS lobectomy by the anterior or posterior approach is a safe, feasible procedure that conforms to the oncological criteria for lung cancer surgery, since—as in conventional surgery—mediastinal lymphadenectomy can be performed simultaneously. With experience accumulated, minimally invasive strategies can be applied to more challenging operations.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Tschernko EM, Hofer S, Bieglmayer C, et al. Early postoperative stress: video-assisted wedge resection/lobectomy vs conventional axillary thoracotomy. Chest 1996;109:1636-42. [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 960-1. [PubMed]
- Pan TW, Wu B, Xu ZF, et al. Video-assisted thoracic surgery versus thoracotomy for non-small-cell lung cancer. Asian Pac J Cancer Prev 2012;13:447-50. [PubMed]
- Jiao W, Zhao Y, Huang T, et al. Two-port approach for fully thoracoscopic right upper lobe sleeve lobectomy. J Cardiothorac Surg 2013;8:99. [PubMed]
- Richards JM, Dunning J, Oparka J, et al. Video-assisted thoracoscopic lobectomy: the Edinburgh posterior approach. Ann Cardiothorac Surg 2012;1:61-9. [PubMed]
- Marty-Ané CH, Canaud L, Solovei L, et al. Video-assisted thoracoscopic lobectomy: an unavoidable trend? A retrospective single-institution series of 410 cases. Interact Cardiovasc Thorac Surg 2013;17:36-43. [PubMed]