Surgical indications and optimization of patients for resectable esophageal malignancies
Overview
The American Cancer Society projects that in 2013, 17,990 adults will obtain a diagnosis of esophageal cancer and 15,210 will succumb to the disease. While the incidence of this cancer is relatively low, the long-term prognosis is grim with overall five-year survival rates of 17%. There are, however, promising avenues for further gain—including earlier detection, aggressive adjuvant therapies, greater patient optimization, and augmented surgical prowess in managing diseases of the esophagus. This is supported by the disparity of survival depending upon the stage of the cancer at the time of diagnosis with isolated esophageal disease harboring a substantial survival benefit in comparison to those with distant metastasis (38% and 3% five-year survival, respectively) (1). This considerable difference has empowered both oncologists and surgeons to work together to uncover the best treatment algorithms with patient-specific paradigms at the forefront of their efforts.
The following review will highlight major risk factors for development of esophageal cancer, recent advancements in the realm of the various imaging modalities utilized in staging, and current trends in medical and surgical management with a focus on patient optimization prior to resection.
Clinical presentation
The unique anatomic structure of the esophagus is the reason most individuals presenting with obstructive esophageal symptoms are diagnosed with advanced disease. Because it lacks a true serosal layer, the smooth muscle of the esophagus can stretch, accommodating a large tumor burden before frank symptomatology is evident. For instance, dysphagia, the most common presenting complaint, is not clinically significant until 50-60% of the esophageal lumen has been comprised by the tumor. Typically, this patient population presents with difficulty in swallowing solid foods which progresses to liquids as the tumor increases further in size. This leads to substantial weight loss, yet another regular complaint in those with esophageal cancer. This baseline state of malnutrition can make chemotherapeutic and surgical recovery difficult and the importance of a knowledgeable nutritional team must not be underemphasized (2).
Additionally, patients may complain of painful swallowing (odynophagia) which can further exacerbate the aforementioned anorexia. The root cause of this discomfort is most likely multifactorial, caused in part by overlying ulceration as well as direct mediastinal invasion. A number of respiratory specific findings, while rare, can represent tumor invasion into local structures. For instance, hoarseness is indicative of laryngeal nerve involvement and a recurrent cough generally represents a fistulous communication between the esophagus and the tracheobronchial tree. The combination of symptoms present at diagnosis depends largely upon the location of the tumor along the length of the esophagus and, therefore, is in part dictated by whether or not the tumor represents adenocarcinoma or squamous cell carcinoma.
Adenocarcinoma and squamous cell carcinoma: an overview, including disease-specific risk factors
When discussing cancer of the esophagus, it is crucial to differentiate between the two major subtypes—adenocarcinoma (ACA) and squamous cell carcinoma (SCC)—as the risk factors and disease specific characteristics differ significantly depending upon the cell-line of origin (3-5). While there remains a higher incidence of squamous cell carcinoma worldwide, the occurrence of adenocarcinoma has increased in the past three decades while the former appears to have been stable or slightly decreased (6,7). Although the root cause of this trend is not completely understood, it does correlate with a more thorough understanding of the pathogenesis of esophageal cancer as well as recent advancements in disease detection.
The most thoroughly investigated and significant risk factor for adenocarcinoma is the presence of gastroesophageal reflux disease (GERD) (8). While there have also been associations between morbid obesity, medications that diminish the basal lower esophageal sphincter tone, long-standing tobacco abuse and previous thoracic radiation and the development of cancer, reflux remains the most influential risk factor in the progression to invasive disease (8-13). The basic premise is that reflux disease results in intestinal metaplasia (Barrett’s esophagus), which further evolves into dysplasia and eventually includes foci of microscopic or grossly invasive cancer. The biology of this degradation includes alterations in various, universally accepted cancer genes, such as p53, p16, APC and telomerase, as well as complex interactions between bile, acid, the Cdx gene and the diagnosis of adenocarcinoma (14-16).
The pathway from normal esophageal mucosa to the appearance of squamous cell carcinoma has not been entirely discerned, but there are, nevertheless, some clear risk factors that have been implicated in the development of SCC. This subtype has been linked both to alcohol consumption and to tobacco abuse, as well as a history of head and neck cancer and previous thoracic radiation. Interestingly, several studies have also established the association between red meat consumption and an increased risk of esophageal SCC, presumably due to the production of N-nitroso compounds, which have proven to be carcinogenic in animal models (17-19).
This conversation is not purely academic in nature, as a greater realization of the pathogenesis and biological progression of both SCC and ACA has afforded surgeons and oncologists with an improved understanding of the natural history of esophageal disease, its aggressivity, and its expected response to treatment. There is a general consensus that irrespective of histological tumor type, an R0 resection (microscopically negative margins) and the presence of lymph node metastasis are prognostic factors in patient’s undergoing surgical intervention for esophageal cancer (3,20). Historically, the studies which have attempted to further delineate prognosis based on subtype have been divided, with several demonstrating a survival benefit in those with ACA (5,21), several presenting improved outcomes in patients with SCC (22,23), and still others showing no significant difference in either group (24).
As the debate surrounding the true impact of histology on overall survival continues, additional disagreement can be found when examining the response of SCC and ADA to neoadjuvant therapies. A large, single institution series demonstrated that after treatment with similar neo-adjuvant regimens, esophageal squamous cell cancer was associated with a higher rate of pCR than adenocarcinoma (42.8% versus 20%) (25). Cancer histology has been reported to influence post-neoadjuvant prognostic characteristics as well. While the most significant predictor of survival in adenocarcinoma appears to be residual nodal status (26), persistent local disease appears to be the most important element in squamous cell cancer (25). Additional studies have established that after trimodal therapy, patients with ACA frequently had malignant lymphadenopathy in the esophageal specimen, which inevitably results in a shorter time-to-metastasis (4,27,28). A more thorough review regarding the impact of neoadjuvant therapy on overall survival will follow in a subsequent section, but it is valuable to discuss the utility of viewing ACA and SCC as separate but similar entities rather than solely grouping them under the modifier ‘esophageal cancer’. While the relevance of these distinctions to the surgeon might not be initially obvious, preoperative optimization is crucial in order to maximize the overall benefit after an esophagectomy.
Diagnosis and staging
Once definitively diagnosed, a combination of imaging modalities has been utilized to most accurately stage esophageal cancer according to the recommendations of the American Joint Commission on Cancer’s 7th edition guidelines, which include its depth of invasion (T), its degree of nodal involvement (N) and its spread to distant sites (M) (29). Accurate staging is instrumental to the treatment team as it dictates which therapeutic approach will become the focus of the management strategy.
Endoscopic ultrasound (EUS) is the preferred mechanism to determine the depth of esophageal invasion with an accuracy of almost 90% (30). Precision in distinguishing between the various ‘T’ stages becomes critical to the surgical team because advances in radiofrequency and endoscopic therapies have afforded patients with dysplastic disease as well as early stage, superficial esophageal cancer (Tis and T1a disease, respectively) an alternative to radical esophagectomy (30-34). Furthermore, local invasion of unresectable structures such as the aorta, vertebrae and the trachea (T4b) excludes surgery as a feasible treatment option. Due to it being widely accepted that the number of positive nodes in the esophagectomy specimen is prognostic of overall survival, it is also essential that imaging be sensitive enough to accurately detect suspicious lymphadenopathy (29,35). Accordingly, in order to be assured of the nodal status preoperatively, an array of information is obtained from EUS, CT, and PET/CT. Aside from merely offering a mechanism for staging, these tools can dictate whether a lesion is amenable to surgical resection. Positive, or highly suspicious, nodes outside of the typical resection field as well as distant sites of metastasis (M1 disease) denote incurable disease with very poor long-term prognosis (1).
Approach to treatment: the interplay of neoadjuvant, endoscopic and surgical therapies
As mentioned previously, individuals with esophageal cancer typically seek medical attention once the disease has progressed to a more advanced stage, resulting in a treatment strategy of which a multi-modal approach is central (36,37). Patients must be carefully analyzed by the surgery, gastroenterology, and oncology services to determine which therapy, or therapies, will be the foundation of this stage-specific approach.
High grade dysplasia (carcinoma in situ)
The risk of progression from high grade dysplasia (HGD) to invasive cancer has been estimated at 10% per year (38). Historically, esophagectomy had been recommended for patients with areas of HGD because it had been associated with a 40-60% risk of harboring cancer (39). More recent investigations, however, have questioned the need for such aggressive treatment in this population of patients, and have demonstrated instead, that radiofrequency ablation (RFA) provides adequate eradication in those with both high- and low-grade dysplasia (90.5% and 81.0%, respectively) (33). Furthermore, in one large study, only 3.6% of patients had disease progression after ablation therapy and an even small number (1.2%) developed cancer (33). Of note, RFA should be avoided in patients with long segments of dysplasia, nodular features or multifocal disease, since these characteristics have been associated with failure of endoscopic treatment.
Stage I disease
As stated above, superficial lesions involving the lamina propria (T1a) without extension into the submucosa (T1b) are typically responsive to endoscopic mucosal resection (EMR) with five-year survival rates above 90% (30-32,34,40,41). This is attributable to the low rate of lymphatic involvement associated with these early stage tumors (42,43). EMR offers a lower-risk alternative to surgical intervention in applicable tumors with complication rates quoted in some studies to be as low as 7% while simultaneously maintaining similar long-term survival as esophagectomy (44,45). The use of endoscopic therapy is cautioned however, in cancers that have invaded beyond the lamina propria (T1b) as locoregional control can be compromised due to a substantial risk of lymph node involvement. These patients, given they have no appreciable metastases, should be referred for a formal resection with lymph node dissection.
Stage II and III disease
The ultimate goal and interest of the surgeon, however, is being able to select which patients will benefit from surgical resection in terms of overall survival and which ones will not. Consequently, patients with advanced, but resectable, lesions should be presented at multi-disciplinary cancer conferences (MDCCs) in order to ensure that the treatment plan follows the most up-to-date and widely accepted guidelines. Several studies have outlined the benefits of MDCCs in patients with esophageal cancer which include enhanced staging (and thus a greater percentage of patients foregoing esophagectomy in favor of endoscopic therapy), an improvement in the time interval from diagnosis to commencement of treatment, and most importantly, a favorable impact on five-year survival (46-48).
These conferences refer to the National Comprehensive Cancer Network (NCCN) guidelines for esophageal cancer as a framework from which to construct their specific intervention. T1b-T4a cancers (tumors invading the submucosa, muscularis propria, adventitia, or specific adjacent structures—pleura, pericardium, and diaphragm) represent resectable disease (49). For those deemed candidates for esophageal resection and especially in T2-T4a and N + (stage II and stage III) disease, preoperative therapy should be considered. This has been supported in several randomized control trials and a large meta-analysis, which have all demonstrated a distinct overall and disease-free survival benefit in individuals undergoing chemoradiotherapy prior to surgical resection (50-52). In the CROSS study, there was a 34% lower risk of death in patients treated with combined neoadjuvant and surgical therapy in comparison to those who only underwent esophagectomy without any prior systemic therapy (52). There is always the theoretic concern that radiation-induced fibrosis will complicate an ensuing resection, but this was not supported in the aforementioned study as nearly 94% of patients underwent successful esophagectomy following chemoradiotherapy (52). Table 1 provides a brief overview of a select number of the randomized-controlled trials evaluating the benefit of induction chemoradiation therapy.
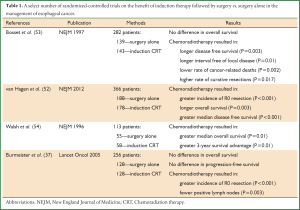
Full table
Stage IV disease
T4b cancers, or those involving the heart, great vessels, trachea or other adjacent organs (e.g., the liver, pancreas, lung and spleen) are not amenable to surgical intervention unless palliation is sought (49). And although there have been several smaller case reports of subsequent metastasectomy in patients with previously resected esophageal cancer primaries, there have been no large trials that have exhibited a survival benefit in surgically treated Stage IV disease and, currently, it cannot be universally recommended (49,55).
Patient optimization prior to surgical resection
Esophagectomy is an incredibly demanding operation for patients with cancer of the thoracic esophagus and subsequent morbidity is common following resection. It is imperative, therefore, aside from merely selecting the most appropriate treatment strategy, that the surgical team consults multidisciplinary services to perform a thorough preoperative assessment of the patient’s cardiac, pulmonary and nutritional reserves.
With gastrointestinal obstruction and tumor cachexia regularly present in esophageal cancer, almost all patients exhibit some degree of malnutrition. There is substantial evidence that malnutrition is immunosuppressive and has a negative impact on survival (56,57). Accordingly, it is generally recommend that placement of definitive enteral access be considered at the time of resection and that patients who are unable to tolerate at least 50% of their goal calories be started on feeds postoperatively (58). While access is usually secured via the jejunum during the esophagectomy, we support the creation of gastric access prior to induction therapy to ensure that preoperative malnutrition is minimized. This can be performed safely via an endoscopic or laparoscopic approach, but careful attention is needed to avoid injury to the gastroepiploic artery, which will supply the gastric conduit during reconstruction.
As mentioned above, patients with relatively more advanced disease are typically treated with a combination of induction chemoradiation followed by a definitive resection. Timing of esophagectomy after preoperative therapy has been under scrutiny as there is a delicate balance between allowing patient recovery while also avoiding progression of disease in the interim. Appropriately, several studies have evaluated this time interval and most centers have now adopted a 6-8-week window after induction therapy during which to schedule an esophagectomy.
Surgical options: trans-thoracic, trans-hiatal, and minimally-invasive approaches
Currently, surgical resection remains the only treatment modality with a chance of oncologic cure in patients with more advanced esophageal carcinoma. The operation can be performed either via a transthoracic or transhiatal approach. The most widely used techniques are the Ivor Lewis esophagectomy (ILE), the transhiatal esophagectomy (THE) and the McKeown esophagectomy. Minimally invasive esophagectomy (MIE) has recently emerged as a cutting edge option for esophageal resection. Using smaller incisions, MIE harnesses the already well-established open techniques while minimizing the physiologic impact of this large-scale operation.
The ILE begins with a laparotomy for conduit construction and mobilization. A right thoracotomy is subsequently conducted for the esophageal and nodal dissection followed by an intrathoracic anastomosis. The McKeown esophagectomy is similar to the ILE however a cervical anastomosis is created via a left neck incision. It is prudent for the surgeon to select a thoracic approach for: tumors abutting the airway or mediastinal vasculature, large middle third esophageal lesions in which a radical resection may prove necessary, patients with suspected mediastinal fibrosis or in patients with prior gastrointestinal surgery which could lead to technical limitations for conduit mobilization to the neck.
THE commences similarly to an ILE with a laparotomy for gastric mobilization and conduit formation while the lymphadenectomy and mediastinal dissection are performed via the diaphragmatic hiatus. The proximal esophagus is subsequently mobilized via a left neck incision and a cervical anastomosis is fashioned. Patients with marginal pulmonary status can benefit from a THE due to the lack of a thoracotomy resulting in a decreased incidence of ventilator dependence and pneumonia (59,60). This is largely attributed to poor pulmonary toileting postoperatively secondary to pain and splinting. Assessing the outcomes of THE versus ILE, the data show that perioperative morbidity and mortality are roughly equivalent. In a 945 patients study, Rentz et al., showed a mortality rate of 10% for ILE and 9.9% for THE (61). Morbidity occurred in 47% of patients following ILE and 49% for THE (61). Risk factors for mortality following an esophageal resection include a serum albumin less than 3.5 g/dL, blood transfusions of more than four units and age greater than 65 (61). There was no difference noted in the incidence of renal failure, infection, pulmonary failure, bleeding or mediastinitis between the two cohorts (61).
Proponents of the thoracic approaches to esophagectomy argue that ILE has been shown to have fewer anastomotic strictures and leaks as compared to THE (62). Moreover it touts a superior oncologic result due to increased exposure, which allows improved visualization of the tumor and the mediastinum resulting in an increased nodal yield as compared to THE. One study showed that ILE collected a mean of 18.5 nodes as compared to 9 for THE (63). Due to the regional lymphatic basins exhibiting a high rate of nodal metastasis, a more substantial nodal yield is important for staging and prognosis. The results of a European randomized study showed that, although median overall, disease-free, and quality-adjusted survival did not differ statistically between patients undergoing transhiatal or transthoracic esophagectomy, there was a trend toward improved long-term survival at five years with the extended transthoracic approach (64). This subject remains controversial since the benefit of an extended lymphadenectomy is often outweighed by the morbidity of the operation. It has become our practice, however, to utilize a thoracotomy incision or a minimally invasive strategy in most every patient to ensure an adequate lymphadenectomy, with THE being reserved for multifocal or long segment, high grade dysplasia or T1a disease not suitable for EMR.
MIE has now emerged as a safe and feasible operation to offer patients at high volume centers without the need for special selection criteria (65-67). A multitude of studies, including several meta-analyses and a randomized, controlled trial, have reported equivalent mortality rates between MIE and open esophagectomy (OE) (66,68-70). Moreover, MIE has been shown to be associated with a decrease in morbidity, duration of mechanical ventilation, as well as in ICU and overall hospital stay (67-70).
While MIE has demonstrated sound perioperative outcomes, it is also noteworthy that the oncologic impact of the operation is not changed when compared to OE. With improved visualization, the median nodal harvest his higher for MIE than OE (66). Additionally, margin status is similar between the two groups with 87% R0 resections in the MIE group as compared to 92% in the OE cohort (66). Disease free and overall survival following MIE are also similar to its open counterpart, although these data reflect only short term follow-up (65-67). Another benefit to MIE is the enhanced quality of life (QOL) following the operation. Zeng et al. showed that following MIE, patients had improved QOL scores, lower pain scores and increased physical function scores (71). Therefore MIE could be the answer for an extensive esophageal and nodal dissection, without the additional morbidity associated with an open, transthoracic approach.
The extent of lymphadenectomy, however, remains a controversial topic. Three field lymphadenectomy, which adds an extensive cervical dissection, is widely advocated by high volume centers in Japan and other countries in the Eastern hemisphere (72-75). Cervical nodal dissection might improve loco-regional control in aggressive upper esophageal lesions, which happen to be more common in these countries (73-75).
However, because the correlation between the three field approach and improved survival is absent, the extended lymphadenectomy has not garnered much support in the West (76). Additionally, there is the concern that a three-field lymph node dissection merely results in stage migration with the added morbidity of a neck dissection. The number of lymph nodes harvested during esophagectomy is likely more important than the field of dissection. Several studies have demonstrated that a greater number of lymph nodes removed at the time of resection is associated with progressively increased survival. This associated, however, is not linear and is believed to be stronger for locally advanced tumors (77-79). It is, therefore, recommended that a minimum of ten nodes be resected for T1 cancers, 20 nodes for T2 cancers and 30 nodes for T3/T4 cancers in order to maximize 5-year survival.
Conclusions
While the diagnosis of esophageal cancer remains devastating, a greater understanding of the disease’s pathogenesis and the impacts of both neoadjuvant and surgical interventions on overall survival have been encouraging. Due to enhancements in the various modalities utilized in esophageal cancer imaging, improved staging has allowed surgeons to select which patient populations will be afforded the greatest benefit after esophageal resection. Similarly, patients with more extensive disease have profited from a combination of more efficacious radiation and chemotherapy prior to their surgical resection. These numerous facets of the treatment platform have been incorporated into multi-disciplinary cancer conferences, which have permitted a rich dialogue between the numerous departments involved in the management of this complex neoplasm. Areas for future investigation include improved screening in high risk individuals, earlier detection by capturing circulating tumor cells or other malignant markers and more personalized treatment paradigms using each individual’s unique genomic targets.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Miller KR, Bozeman MC. Nutrition therapy issues in esophageal cancer. Curr Gastroenterol Rep 2012;14:356-66. [PubMed]
- Mariette C, Finzi L, Piessen G, et al. Esophageal carcinoma: prognostic differences between squamous cell carcinoma and adenocarcinoma. World J Surg 2005;29:39-45. [PubMed]
- Rohatgi PR, Swisher SG, Correa AM, et al. Histologic subtypes as determinants of outcome in esophageal carcinoma patients with pathologic complete response after preoperative chemoradiotherapy. Cancer 2006;106:552-8. [PubMed]
- Siewert JR, Stein HJ, Feith M, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg 2001;234:360-7; discussion 368-9. [PubMed]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [PubMed]
- Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005;97:142-6. [PubMed]
- Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31. [PubMed]
- García Rodríguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut 2006;55:1538-44. [PubMed]
- Lagergren J, Bergström R, Adami HO, et al. Association between medications that relax the lower esophageal sphincter and risk for esophageal adenocarcinoma. Ann Intern Med 2000;133:165-75. [PubMed]
- Lagergren J, Bergström R, Adami HO, et al. Association between medications that relax the lower esophageal sphincter and risk for esophageal adenocarcinoma. Ann Intern Med 2000;133:165-75. [PubMed]
- Merry AH, Schouten LJ, Goldbohm RA, et al. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut 2007;56:1503-11. [PubMed]
- Nilsson M, Johnsen R, Ye W, et al. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA 2003;290:66-72. [PubMed]
- Clemons N, Phillips W, Lord RV. Signaling pathways in the molecular pathogenesis of adenocarcinomas of the esophagus and gastresophageal junction. Cancer Biol Ther 2013;14. [Epub ahead of print]. [PubMed]
- González MV, Artímez ML, Rodrigo L, et al. Mutation analysis of the p53, APC, and p16 genes in the Barrett’s oesophagus, dysplasia, and adenocarcinoma. J Clin Pathol 1997;50:212-7. [PubMed]
- Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol 2008;295:G211-8. [PubMed]
- Choi Y, Song S, Song Y, et al. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol 2013;19:1020-9. [PubMed]
- Salehi M, Moradi-Lakeh M, Salehi MH, et al. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev 2013;71:257-67. [PubMed]
- Cross AJ, Freedman ND, Ren J, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol 2011;106:432-42. [PubMed]
- Lerut T, De Leyn P, Coosemans W, et al. Surgical strategies in esophageal carcinoma with emphasis on radical lymphadenectomy. Ann Surg 1992;216:583-90. [PubMed]
- Giuli R, Gignoux M. Treatment of carcinoma of the esophagus. Retrospective study of 2,400 patients. Ann Surg 1980;192:44-52. [PubMed]
- Lund O, Hasenkam JM, Aagaard MT, et al. Time-related changes in characteristics of prognostic significance in carcinomas of the oesophagus and cardia. Br J Surg 1989;76:1301-7. [PubMed]
- Mathisen DJ, Grillo HC, Wilkins EW Jr, et al. Transthoracic esophagectomy: a safe approach to carcinoma of the esophagus. Ann Thorac Surg 1988;45:137-43. [PubMed]
- Lieberman MD, Shriver CD, Bleckner S, et al. Carcinoma of the esophagus. Prognostic significance of histologic type. J Thorac Cardiovasc Surg 1995;109:130-8; discussion 139. [PubMed]
- Rizk NP, Seshan VE, Bains MS, et al. Prognostic factors after combined modality treatment of squamous cell carcinoma of the esophagus. J Thorac Oncol 2007;2:1117-23. [PubMed]
- Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol 2007;25:507-12. [PubMed]
- Rohatgi P, Swisher SG, Correa AM, et al. Characterization of pathologic complete response after preoperative chemoradiotherapy in carcinoma of the esophagus and outcome after pathologic complete response. Cancer 2005;104:2365-72. [PubMed]
- Rohatgi PR, Swisher SG, Correa AM, et al. Comparison of clinical stage, therapy response, and patient outcome between squamous cell carcinoma and adenocarcinoma of the esophagus. Int J Gastrointest Cancer 2005;36:69-76. [PubMed]
- Esophagus and esophagogastric junction. In: Edge SB, Byrd DR, Compton CC, et al. eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010:103-11.
- Rösch T. Endosonographic staging of esophageal cancer: a review of literature results. Gastrointest Endosc Clin N Am 1995;5:537-47. [PubMed]
- Endo M. Endoscopic resection as local treatment of mucosal cancer of the esophagus. Endoscopy 1993;25:672-4. [PubMed]
- Nishimaki T, Tanaka O, Suzuki T, et al. Tumor spread in superficial esophageal cancer: histopathologic basis for rational surgical treatment. World J Surg 1993;17:766-71; discussion 771-2. [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [PubMed]
- Yoshinaka H, Shimazu H, Fukumoto T, et al. Superficial esophageal carcinoma: a clinicopathological review of 59 cases. Am J Gastroenterol 1991;86:1413-8. [PubMed]
- Chen J, Xu R, Hunt GC, et al. Influence of the number of malignant regional lymph nodes detected by endoscopic ultrasonography on survival stratification in esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2006;4:573-9. [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [PubMed]
- Reid BJ, Levine DS, Longton G, et al. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol 2000;95:1669-76. [PubMed]
- Pellegrini CA, Pohl D. High-grade dysplasia in Barrett’s esophagus: surveillance or operation? J Gastrointest Surg 2000;4:131-4. [PubMed]
- Inoue H. Endoscopic mucosal resection for esophageal and gastric mucosal cancers. Can J Gastroenterol 1998;12:355-9. [PubMed]
- Katada C, Muto M, Momma K, et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy 2007;39:779-83. [PubMed]
- Matsubara T, Ueda M, Abe T, et al. Unique distribution patterns of metastatic lymph nodes in patients with superficial carcinoma of the thoracic oesophagus. Br J Surg 1999;86:669-73. [PubMed]
- Nagawa H, Kaizaki S, Seto Y, et al. The relationship of macroscopic shape of superficial esophageal carcinoma to depth of invasion and regional lymph node metastasis. Cancer 1995;75:1061-4. [PubMed]
- Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery 1998;123:432-9. [PubMed]
- Shimizu Y, Tsukagoshi H, Fujita M, et al. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc 2002;56:387-90. [PubMed]
- Davies AR, Deans DA, Penman I, et al. The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro-esophageal cancer. Dis Esophagus 2006;19:496-503. [PubMed]
- Freeman RK, Van Woerkom JM, Vyverberg A, et al. The effect of a multidisciplinary thoracic malignancy conference on the treatment of patients with esophageal cancer. Ann Thorac Surg 2011;92:1239-42; discussion 1243. [PubMed]
- Stephens MR, Lewis WG, Brewster AE, et al. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis Esophagus 2006;19:164-71. [PubMed]
- National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers Version 2.2013 [database online]. Insert City of Publication Here see notes: 6 May 2013. Assessed 14 July 2013. Available online: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Malthaner RA, Collin S, Fenlon D. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev 2006;CD001556. [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Chen F, Sato K, Sakai H, et al. Pulmonary resection for metastasis from esophageal carcinoma. Interact Cardiovasc Thorac Surg 2008;7:809-12. [PubMed]
- Dionigi P, Dionigi R, Nazari S, et al. Nutritional and immunological evaluations in cancer patients. Relationship to surgical infections. JPEN J Parenter Enteral Nutr 1980;4:351-6. [PubMed]
- Dominioni L, Rovera F, Pericelli A, et al. The rationale of early enteral nutrition. Acta Biomed 2003;74 Suppl 2:41-4. [PubMed]
- Mariette C, De Botton ML, Piessen G. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol 2012;19:2128-34. [PubMed]
- Bakhos CT, Fabian T, Oyasiji TO, et al. Impact of the surgical technique on pulmonary morbidity after esophagectomy. Ann Thorac Surg 2012;93:221-6; discussion 226-7. [PubMed]
- Bhayani NH, Gupta A, Dunst CM, et al. Esophagectomies with thoracic incisions carry increased pulmonary morbidity. JAMA Surg 2013;148:733-8. [PubMed]
- Rentz J, Bull D, Harpole D, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg 2003;125:1114-20. [PubMed]
- Rindani R, Martin CJ, Cox MR. Transhiatal versus Ivor-Lewis oesophagectomy: is there a difference? Aust N Z J Surg 1999;69:187-94. [PubMed]
- Wolff CS, Castillo SF, Larson DR, et al. Ivor Lewis approach is superior to transhiatal approach in retrieval of lymph nodes at esophagectomy. Dis Esophagus 2008;21:328-33. [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [PubMed]
- Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed]
- Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg 2012;147:768-76. [PubMed]
- Schwameis K, Ba-Ssalamah A, Wrba F, et al. The implementation of minimally-invasive esophagectomy does not impact short-term outcome in a high-volume center. Anticancer Res 2013;33:2085-91. [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci 2010;55:3031-40. [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [PubMed]
- Zeng J, Liu JS. Quality of life after three kinds of esophagectomy for cancer. World J Gastroenterol 2012;18:5106-13. [PubMed]
- Sannohe Y, Hiratsuka R, Doki K. Lymph node metastases in cancer of the thoracic esophagus. Am J Surg 1981;141:216-8. [PubMed]
- Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991;48:411-20. [PubMed]
- Shimada H, Nabeya Y, Matsubara H, et al. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg 2006;191:250-4. [PubMed]
- Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72; discussion 972-4. [PubMed]
- Orringer MB, Marshall B, Stirling MC. Transhiatal esophagectomy for benign and malignant disease. J Thorac Cardiovasc Surg 1993;105:265-76; discussion 276-7. [PubMed]
- Rizk N, Venkatraman E, Park B, et al. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2006;132:1374-81. [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer 2008;112:1239-46. [PubMed]


