Why did VALIDATE-SWEDEHEART not validate the results of HORIZONS?
Intravenous anticoagulation is recommended during percutaneous coronary intervention (PCI) in all patients with acute coronary syndromes to avoid ischemic and embolic complications during the procedure and improve outcome (1). On the other hand intravenous anticoagulation is associated with an increase in bleeding complications, which have an adverse impact on prognosis (2). Therefore antithrombotic regimens balancing the risk of ischemia and bleeding are preferably (1). Unfractionated heparin (UFH) has been the first available intravenous anticoagulant and therefore traditionally used in PCI. However, UFH has several pharmacologic limitations including a high intra- and inter-individual variability. Bivalirudin is a thrombin-specific anticoagulant, which has the ability to inactivate both free and fibrin-bound thrombin. In several randomized clinical trials bivalirudin has demonstrated efficacy and safety in patients undergoing PCI in the elective patients, NSTE-ACS and ST segment elevation myocardial infarction (STEMI) (3-11). In the HORIZONS trial, bivalirudin reduced the primary combined endpoint including death, myocardial infarction and bleeding complications compared to heparin plus routine glycoprotein IIb/IIIa inhibitors (GPI) in patients undergoing primary PCI for STEMI (3). Moreover cardiovascular mortality was reduced after 30 days and at 3-year follow-up, both in patients with and without bleeding complications (12). Subsequent trials reported a consistent reduction of bleeding complications compared to UFH with or without the routine use of GP IIb/IIIa inhibitors, both in patients with STEMI and NSTE-ACS (4-11). In most studies in STEMI a concern was the higher rate of early stent thrombosis occurring within the first hours after PCI.
Just recently the results of the VALIDATE-SWEDEHEART trial were published, which compared bivalirudin versus UFH in patients undergoing PCI for STEMI or NSTEMI (13). The primary combined endpoint was all-cause mortality, reinfection or major bleeding until 6 months after the procedure. The trial used the platform of the SWEDEHEART-Registry and enrolled 6,005 patients, of these 50% had STEMI. Patients with an intended use of GP IIb/IIIa inhibitors were excluded, so that the higher risk patients were not enrolled into the trial. Most of the patients had radial access (90%) and were treated with ticagrelor (85%). There were not differences between the treatments for the primary combined endpoint, any of the individual components or other endpoints such as stroke and stent thrombosis. The 6-month mortality was low with 1.9% and 1.7% in the two groups. The authors report several limitations of their trial including its open design, the low rate of ischemic events and the follow-up performed by phone calls. Surprisingly there was no difference in major bleeding complications. While the authors speculate that this was due to a high rate of transracial procedures and the low rate of GP IIb/IIIa inhibitors (3%), another reason could be that most patients treated with bivalirudin received UFH (mean dose 3,470 IU) as well, masking the advantage of bivalirudin. In addition the trial was not powered to examine bleeding rates only and at 30 days which is the preferred time-point to determine the safety and efficacy of periprocedural anticoagulation. In contrast to earlier findings there was no increased risk of early stent thrombosis with bivalirudin, which might be explained by the high rate and early use of potent P2Y12 receptor ant antagonists and the prolonged infusion of bivalirudin in two thirds of the patients. One important point is that more than 50% of the screened population has not been included into the trial, especially those with higher ischemic or bleeding risk, so the generalizability of findings are limited.
Since STEMI is associated with greater platelet activation and larger thrombus burden as well as with larger infarcts antithrombotic therapy seems more important and have a greater impact on outcome than in NSTEMI. In a meta-analysis of 6 randomized trials with over 13,000 patients with STEMI bivalirudin compared to UFH has been associated with a reduction in major bleeding, an increase in acute stent thrombosis and a reduction in cardiac and total mortality (14). The benefit in bleeding seemed to be influenced by the rate of radial access and the avoidance of routine use of GP IIb/IIIa inhibitors. In Figure 1 the rate of major bleedings in randomized trials in STEMI according to the concomitant use of GP IIb/IIIa inhibitors is shown. The comparative mortality rates are from the same trials is summarized in Figure 2. However, the mortality reduction with bivalirudin observed in the HORIZONS trial could not be replicated in 4 of the 5 subsequent randomized trials in STEMI. The reasons are multifactorial and include lower use of GP IIb/IIIa inhibitors, higher use of radial access (both associated with lower bleeding complications), different patient populations and study protocols.
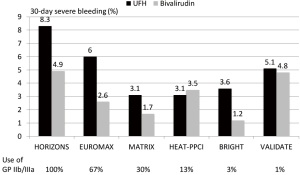
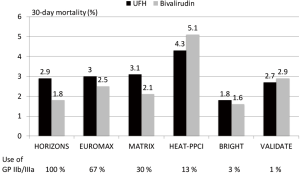
A new meta-analysis incorporating the individual patient data of all trials seems warranted to identify subgroups which might benefit more from either UFH or bivalirudin.
So far the following recommendations seem reasonable for clinical practice. For intravenous anticoagulation in patients with STEMI undergoing primary PCI an individualized approach seems warranted. Clearly in patients with an increased risk of bleeding complications (e.g., high CRUSADE score, previous bleeding, older age, femoral access with larger sheets) bivalirudin should be preferred. To reduce the risk of early stent thrombosis it should be combined with the early use of the newer more potent P2Y12 inhibitors prasugrel and ticagrelor. Especially in patients with high thrombus burden the infusion of the PCI dose of bivalirudin should be prolonged for 2–4 hours after the procedure. Given the much higher price of bivalirudin in patients with low ischemic and bleeding risk UFH with a bolus of 70–100 IU/kg seems to be the most appropriate and cost effective treatment. Another alternative is the low-molecular weight heparin enoxaparin given with a single bolus of 0.5 mg/kg, which was associated with lower ischemic complications compared to UFH in the randomised ATOLL trial (15). The concomitant use of GP IIb/IIIa inhibitors should be limited to patients with high ischemic risk (e.g., high thrombus burden), presenting early and low bleeding risk (16).
Acknowledgements
None.
Footnote
Conflicts of Interest: AstraZeneca, B. Braun, BMS, BoehringerIngelheim, BayerHealtcare, Correvio, Daiichi Sankyo, Eli Lilly, Medtronic, Medicines Company, MSD, Novartis, Pfizer, Sanofi.
References
- Zeymer U, Rao SV, Montalescot G. Anticoagulation in coronary intervention. Eur Heart J 2016;37:3376-85. [Crossref] [PubMed]
- Steg PG, Huber K, Andreotti F, et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J 2011;32:1854-64. [Crossref] [PubMed]
- Lincoff AM, Bittl JA, Harrington RA, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003;289:853-63. [Crossref] [PubMed]
- Stone GW, McLaurin BT, Cox DA, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006;355:2203-16. [Crossref] [PubMed]
- Kastrati A, Neumann FJ, Schulz S, et al. Abciximab and heparin versus bivalirudin for non-ST elevation myocardial infarction. N Engl J Med 2011;365:1980-89. [Crossref] [PubMed]
- Stone GW, Witzenbichler B, Guagliumi G, et al. HORIZONS Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008;358:2218-30. [Crossref] [PubMed]
- Steg PG, van 't Hof A, Hamm CW, et al. Bivalirudin started during emergency transport for primary PCI. N Engl J Med 2013;369:2207-17. [Crossref] [PubMed]
- Zeymer U, van’t Hof A, Adgey J, et al. Bivalirudin is superior to heparins alone with bailout GP IIb/IIIa inhibitors in patients with ST-segment elevation myocardial infarction transported emergently for primary percutaneous coronary intervention: a pre-specified analysis from the EUROMAX trial. Eur Heart J 2014;35:2460-67. [Crossref] [PubMed]
- Shahzad A, Kemp I, Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 2014;384:1849-58. [Crossref] [PubMed]
- Han Y, Guo N, Zheng Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA 2015;313:1336-46. [Crossref] [PubMed]
- Leonardi S, Frigoli E, Rothenbühler M, et al. Bivalirudin or unfractionated heparin in patients with acute coronary syndromes managed invasively with and without ST elevation (MATRIX): randomised controlled trial. BMJ 2016;354:i4935. [Crossref] [PubMed]
- Stone GW, Clayton T, Deliargyris EN, et al. Reduction in cardiac mortality with bivalirudin in patients with and without major bleeding: The HORIZONS-AMI trial (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction). J Am Coll Cardiol 2014;63:15-20. [Crossref] [PubMed]
- Erlinge D, Omerovic E, Fröbert O, et al. Bivalirudin versus heparin monotherapyin acute myocardial infarction. N Engl J Med 2017;377:1132-42. [Crossref] [PubMed]
- Shah R, Rogers KC, Matin K, et al. An updated comprehensive meta-analysis of bivalirudin vs heparin use in primary percutaneous coronary intervention. Am Heart J 2016;171:14-24. [Crossref] [PubMed]
- Montalescot G, Zeymer U, Silvain J, et al. Intravenous enoxaparin or unfractionated heparin in primary percutaneous coronary intervention for ST-elevation myocardial infarction: the international randomised open-label ATOLL trial. Lancet 2011;378:693-703. [Crossref] [PubMed]
- De Luca G, Navarese E, Marino P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur Heart J 2009;30:2705-13. [Crossref] [PubMed]


