Evaluation of the 95% limits of agreement of the volumes of 5-year clinically stable solid nodules for the development of a follow-up system for indeterminate solid nodules in CT lung cancer screening
Introduction
With the implementation of computed tomography (CT) lung cancer screening, numerous indeterminate nodules have been detected and their follow-up is crucial. Among several CT lung cancer screening projects, the Dutch-Belgian randomized lung cancer screening trial (NELSON) introduced the management of lung nodules based on volume doubling times (VDT) derived from nodule volumetry using computer software (1-3). Briefly, the volume of solid nodules determined the screening result: <50 mm3 was considered negative, 50–500 mm3 was considered indeterminate, and >500 mm3 was considered positive; if the volume growth (percent volume change) was <25%, the screening result was considered negative, and if the volume growth was ≥25%, the VDT of the nodule was calculated and a VDT <400 days was considered to be a positive result (4). In contrast to NELSON, the National Lung Screening Trial (NLST) used nodule diameter to assess nodule size; participants with nodules of 4 mm or larger in maximal diameter were considered positive (5). Although many volumetry studies have evaluated pulmonary solid nodules (6-18), the standard for the follow-up of pulmonary solid nodules in CT screening in the US and in daily clinical practice is still the measurement of pulmonary nodule size using electronic calipers (19,20) . A review article concluded that accumulating evidence indicates that semi-automatic volume measurements have a higher accuracy and reproducibility than diameter measurements (21). To our knowledge, only one article has reported volume changes in clinically stable solid nodules with long-term CT follow-up (14). To develop a dedicated follow-up system for solid nodules, we attempted to evaluate the 95% limits of agreement for volume changes in nodules that had been clinically stable for 5 years in not only smokers, but also in never smokers as the first step in the developing of such a system. Regarding the assessment of pulmonary solid nodule volumes, several methods have been reported, such as the percent change (6,17), proportional change (17), growth rate (14), and monthly volumetric growth index (6). In the present study, we aimed to evaluate the volume change using the following three methods: the percent change, the proportional change, and the growth rate.
The purpose of this study was to evaluate the 95% limits of agreement for the volumes of 5-year clinically stable solid nodules for the development of a follow-up system for indeterminate solid nodules.
Methods
CT scanning and image reconstruction in CT lung cancer screening
The CT lung cancer screening protocol of the Research Center for Cancer Prevention and Screening has been described elsewhere (22). The scanning protocol from February 2004 to June 2010 was as follows: tube potential, 120 kVp; tube current, 30 mA; collimation, 1 mm × 16 rows; 0.5 second per rotation; pitch, 0.69; standard reconstruction kernel. The scanning protocol after July 2010 was the same for the tube potential, tube current, and rotation speed, but the collimation was 1 mm × 32 rows. CT images were reconstructed using 5 mm thick sections obtained at 5 mm intervals and 2 mm thick sections obtained at 2 mm intervals between February 2004 and November 2011; after December 2011, the CT images were reconstructed using 5 mm thick sections obtained at 5 mm intervals and 1 mm thick sections obtained at 1 mm intervals. If a solid nodule examined using volumetry in this study had been scanned before December 2011, the CT images of the solid nodule were reconstructed in contiguous 1 mm thick sections using the raw CT data.
Patients with 5-year stable nodules
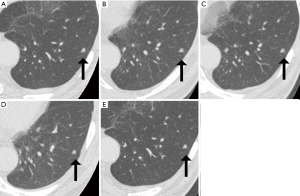
To evaluate the 95% limits of agreement for the volumes of 5-year stable solid nodules, patients with 5-year stable solid nodules with a longest diameter of between 5 mm or larger and less than 10 mm were selected sequentially among a baseline cohort of CT lung cancer screening cases at the Research Center for Cancer Prevention and Screening between February 02, 2004, and March 31, 2007. Information on the smoking status was obtained using a questionnaire at the time of the baseline CT screening. The location of each nodule (lung lobe and distance from the pleura), the shape of the nodule (oval or polygonal), and the presence of contacting vessels were documented. A representative solid nodule imaged at five time points during a 5-year period is shown in Figure 1.
Volumetry of pulmonary solid nodules
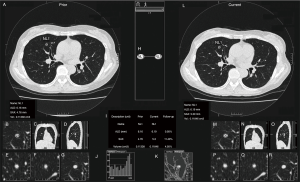
Volume measurement of the solid nodules was performed using commercially available software [Lesion Management Solutions (LMS), MEDIAN Technologies, Valbonne, France], as described elsewhere (23). In brief, the software can detect, segment, and quantify pulmonary solid nodules; after segmentation, the longest diameter, perpendicular diameter, and volume of each nodule were extracted automatically (Figure 2). Readers were able to make manual adjustments to the contour of the lesion as necessary. One thoracic radiologist (Kakinuma R, 33 years’ experience in reading chest CT) measured the volumes of all the solid nodules.
Methods for evaluating volume change
Percent change
The percent change between scans at two time points was calculated as the ratio of the difference in the second volume estimate (V2) and the first volume estimate (V1) relative to the first volume estimate (6,17).
Percent change (%) = 100 × (V2 - V1) / V1
Proportional change
The proportional change was calculated as the ratio of the difference between the V2 and the V1 to the average between the first and second volume estimates (17).
Proportional change (%) = 100 × (V2 - V1) / [(V2 + V1) / 2]
Growth rate
The growth rate measured at any two points in time (T1, T2) was computed as follows (14).
Growth rate (%) = 100 × (V2 - V1) / [V1 (T2 - T1)]
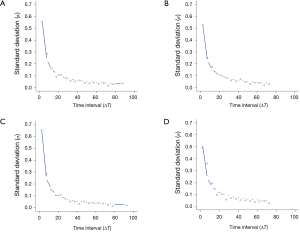
A power function, 𝜎 = a(∆T)-b, with two free parameters, a and b, was used to model the dependence of the standard deviation (SD) (𝜎) of the growth rate on the time interval (∆T = T2 - T1) (14).
Intra- and Inter-reader variability of nodule volume measurements
One thoracic radiologist (Kakinuma R) re-measured the volumes of 52 solid nodules that had been randomly selected from the study cohort at an interval of 1 month. The other radiologist (Muramatsu Y, 37 years of experience) independently measured the volumes of the 52 solid nodules. The intra-reader (Kakinuma R) and inter-reader variability (Kakinuma R vs. Muramatsu Y) were evaluated with Bland-Altman methods.
Evaluation of an increase or decrease in volume of solid nodules based on the 95% limits of agreement for the volumes of 5-year stable solid nodules
A clinical diagnosis of whether a nodule has shown growth or not is based on manual diameter measurements (21). The cutoff-value for evaluating an increase or decrease in the longest diameter in a clinical setting was set as 1.73 mm in the present study, based on the 95% limits of small non-calcified pulmonary nodules (24). For a “clinical diagnosis” in the present study, nodule stability was defined as a difference of less than 1.73 mm in the longest diameter of a solid nodule between the baseline screening CT examination and the final repeat screening CT examination. The longest diameter and the perpendicular diameter were measured using electronic calipers and the results were entered into a nodule database prospectively during the screening process. The 5-year stability of the nodules was determined retrospectively based on the data in the database. Images of each nodule that had been obtained at multiple time points were reviewed by a thoracic radiologist (Kakinuma R) to confirm stability.
To evaluate the increase or decrease in volume, solid nodules with a longest diameter of 5 mm or larger but less than 10 mm, other than 5-year stable solid nodules that showed an increase or decrease in diameter, were chosen sequentially from the baseline cohort of subjects undergoing CT lung cancer screening at the Research Center for Cancer Prevention and Screening between February 02, 2004, and March 31, 2010 (the period for selecting solid nodules with an increase or decrease in diameter was longer than the period for selecting 5-year stable solid nodules because the number of solid nodules with an increase or decrease in diameter was relatively small). An increase or decrease in volume was evaluated using the 95% limits of agreement for each evaluation method.
Statistical analysis
The median and interquartile range (IQR) for age, and the volumes were calculated; the medians of the volumes at baseline for the four smoking statuses were evaluated using the Kruskal-Wallis rank sum test. The mean number and median number of follow-up CT examinations per patient were calculated. The means of days until change detection in the solid nodules were calculated. A Bland-Altman analysis was performed to assess the 95% limits of agreement of the volume changes determined using each evaluation method (25). The relationship between the SD of the growth rate and a power of the time interval was evaluated. The agreement between readers was assessed for the measured volumes using an intraclass correlation coefficient (ICC). The time of the detection of a change between the volume and diameter was evaluated using the Mann-Whitney U test. R software, version 3.1.2 (The R Foundation, Vienna, https://www.r-project.org) was used for the statistical analysis. A P value less than 0.05 was considered statistically significant.
Results
Characteristics of the patients
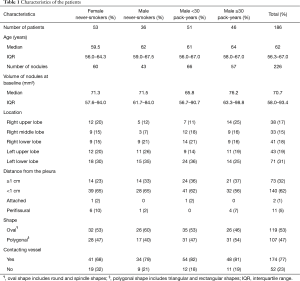
The total numbers of patients and pulmonary solid nodules with a longest diameter between 5 mm or larger and less than 10 mm were 243 and 305, respectively. Among them, 79 solid nodules in 57 patients were less than 50 mm3 after volume measurement; solid nodules with a volume of less than 50 mm3 were excluded from this study because such lesions were considered to be negative results in the NELSON trial. Therefore, 186 patients (median age: 62 years old, and IQR: 56.3–67 years old), and 226 solid nodules (median volume: 70.7 mm3, IQR: 58.0–93.4 mm3) were analyzed in this study. Long-term CT follow-up was performed for a mean of 5.3±0.5 years (range, 5–8.1 years; median, 5.2 years) after the baseline examination. The mean number of follow-up CT examinations per patient was 4.0±0.6 examinations (range, 3–6 examinations; median, 4 examinations). Among the 186 patients, 53 females and 36 males were never-smokers, 51 males were smokers with less than 30 pack-years, and 46 males were smokers with 30 pack-years or more. The volumes of the nodules at baseline, the locations of the nodules, the distances from the pleura, and the shapes of the nodules are shown in Table 1. The medians of the volumes of nodules at baseline for the four smoking statuses were not significantly different (Kruskal-Wallis chi-squared =2.3174, df =3, P=0.5092). Seventy-seven percent (174 out of 226) of the solid nodules were in contact with pulmonary vessels.
Full table
The 95% limits of agreement for volumes in 5-year stable solid nodules
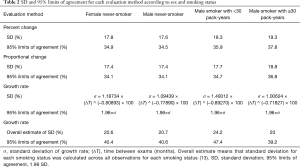
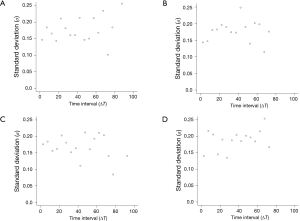
The 95% limits of agreement for volumes in 5-year stable solid nodules are shown in Table 2: range of percent change, from ±34.5% to ±37.8%; range of proportional change, from ±34.1% to ±36.8%; and range of growth rate in overall estimate [“overall estimate” means that the SD was calculated across all observations (14)], from ±39.2% to ±47.4%. The SD of the growth rate was well approximated by a power of the time interval (Figure 3), while the SD of the percent change and the proportional change were independent of the time interval (Figures 4,5).
Full table

The differences in the mean of the volume change between female never-smokers and patients with other smoking statuses were not statistically significant, although the range of the 95% limits of agreement in the male smokers with 30 pack-years or more was slightly larger than those in the patients with other smoking statuses (Tables 2,S1,S2).
Full table
Full table
Evaluation of an increase or decrease in volume of solid nodules based on the 95% limits of agreement for the volumes of 5-year stable solid nodules
The results for ten nodules evaluated in ten patients are shown in Tables 3-6. The age of the patients ranged from 41 to 68 years old (median, 65.5 years old); the smoking statuses were female never-smoker (n=1), male never-smoker (n=3), male smoker with <30 pack-years (n=2), and male smoker with ≥30 pack-years (n=4). Median volume of the ten solid nodules at the baseline examination was 166.6 mm3 (IQR, 77.1–213.8 mm3). Among the six nodules that showed an increase in diameter, two nodules (No. 9 and 10) were resected and diagnosed as adenocarcinomas. The remaining eight nodules were not resected and had not been diagnosed as lung cancers as of March 2014. Although 4 nodules (No.1, 3, 4, 5) did increase in size, we suspected that these nodules were benign nodules. Among the ten nodules, the 95% limits of agreement for the percent change, the proportional change, and the growth rate enabled volume changes to be detected in ten, nine, and ten nodules, respectively. The numbers of nodules detected at an earlier stage than the clinical diagnosis (i.e., an increase or decrease in diameter) were 8, 5, and 7 nodules when evaluated based on the percent change, the proportional change, and the growth rate, respectively (Table 6). The percent change-based, proportional change-based and growth rate-based diagnoses of an increase or decrease in the solid nodules were made at a mean of 302±402 (n=10), 367±455 (n=9), and 329±496 days (n=10) from the baseline scan, respectively, whereas the clinical diagnosis was made at 809±616 days (n=10) (P<0.05, Mann-Whitney U test) (Table 6).
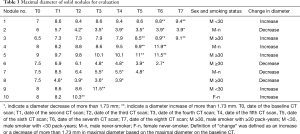
Full table
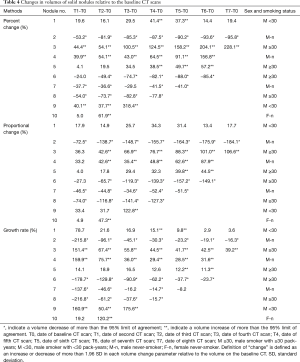
Full table
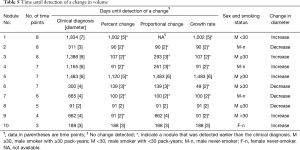
Full table

Full table
Intra-reader variability based on percent and proportional changes
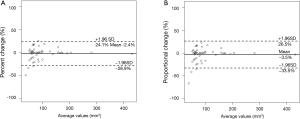
One thoracic radiologist (Kakinuma R) measured the volumes of 52 nodules in 41 patients twice at an interval of 1 month. The 95% limits of agreement were as follows: −2.4±26.5% for percent change; −3.5±30.0% for proportional change (Figure 6); the ICC was 0.990 (95% CI, 0.982–0.994).
Inter-reader variability based on percent and proportional changes
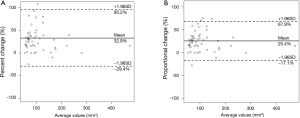
Two radiologists (Kakinuma R, Muramatsu Y) measured the volumes of 52 nodules in 41 patients within the study cohort for the evaluation of inter-reader variability. The 95% limits of agreement were as follows: 32.9%±62.3% for percent change; 25.4%±42.5% for proportional change (Figure 7); the ICC was 0.869 (95% CI, 0.783–0.922).
Discussion
The present study examined whether the 95% limits of agreement for volume changes in solid nodules that were stable for 5 years differed when software other than that used in the NELSON trial was applied to detect nodule changes earlier than that possible using diameter measurements, such as in the NLST. The results showed that the 95% limits of agreement for volume changes in 5-year stable solid nodules may enable the detection of an increase or decrease in solid nodules at an earlier stage than that enabled by a clinical diagnosis
In Japan, lung cancer screening is presently conducted using chest X-rays for population-based screening (26), and low-dose CT lung cancer screening is conducted as an opportunistic screening for not only smokers, but also never-smokers. Lung cancer deaths in never-smokers rank as the fifth most common cause of death in men and the third in women, reflecting a relatively high estimated rate (31% of male patients and 80% of female patients) of lung cancers that are unrelated to smoking in Japan (27). Therefore, the present study evaluated the 95% limits of agreement of the volumes of 5-year clinically stable solid nodules not only in smokers, but also in never-smokers.
With respect to CT scans for the volumetry of solid nodules, several protocols have been reported as follows: one standard-dose CT scan (7,16); two standard-dose CT scans (6); three standard-dose CT scans (9); one low-dose CT scan (10); two low-dose CT scans (8,11); three low-dose CT scans (12); a range from two to seven low-dose CT scans (14); one standard-dose CT scan and one ultra-low-dose CT scan (13); one low-dose CT scan and one ultra-low-dose CT scan (18). The present study used several low-dose CT scans (range, 3–6 scans).
Regarding the CT scan intervals for the volumetry of solid nodules, same-day CT scans (8,9,11,13,17,18) and different-day CT scans (6,12,14,16) have been reported: the shortest intervals were within 10 minutes (8,11,13) and the longest interval was 8.5 years (14). The smallest inter-scan variability of the volume change for same-day CT scans ranged from −20% to 20.4% (18), whereas the largest inter-observer variability for same-day CT scans (two scans within 15 minutes) was 7.4%±44.2% (mean percent difference ± SD) (17).
In the NELSON trial, nodule growth was defined as a change in the volume of at least 25% between two subsequent examinations based on validation studies with repeated low-dose CT examinations performed on the same days, in which the measurement error was maximally 25% (3). However, optimization of the VDT cutoff for fast-growing nodules in lung cancer screening revealed that lowering the VDT cutoff could reduce false-positive referrals (28).
Not all CT screening facilities can use the software that was used in the NELSON trial. Comparison of three software systems for semi-automatic volumetry of pulmonary nodules showed that significant difference was found in measured volume between software in the NELSON trial and the other two software packages (29). The software that we used in the presently reported study has been used and validated in clinical trials (23), but the 95% limits of agreement for the volumes of solid nodules detected during CT screening have never been evaluated. Therefore, we performed the presently reported study.
Which method is more appropriate for evaluating volume changes when developing a follow-up system for indeterminate solid nodules? Although each of the evaluation methods can be implemented using computer software, the percent change might be optimal for implementation in clinical settings because of its simple cutoff value and the finding that in our very small cohort, the number of earlier-detected nodules was larger when the percent change method was used, compared with the other methods that were evaluated. Moreover, the mean number of days until a change was detected was shorter for the percent change method than for the other methods; however, the mean number of days until a change was detected was not significantly different for the percent change method, compared with the other evaluation methods. A prospective study is warranted.
The present study had several limitations. First, only one radiologist (Kakinuma R) retrospectively reviewed the solid nodules on serial CT images, confirmed the clinical stability of the nodules, and performed the volumetry studies for the solid nodules. The radiologist had 33 years of experience in reading chest CT images and 22 years of experience in reading lung cancer screening CT images, although an inherent intra-reader variability exists for the measurement of nodules. Second, although this study utilized a semiautomated quantification of the solid nodule volumes, manual correction of the contour of a nodule was allowed for better segmentation of the nodule because 77% of the solid nodules were in contact with pulmonary vessels; this procedure might have affected the results of this study. Other studies have also reported results with manual correction (14,30). Third, the NELSON lung nodule management system used the percent volume change and the VDT for assessments (1-3). However, the present study did not evaluate the VDTs because of the very small cohort that was used for the evaluation. Fourth, female smokers were not included in this study because the number of female smokers was very small. Finally, regarding the evaluation of the progression of indeterminate solid nodules, the number of solid nodules that showed an increase in the longest diameter was very small because the number of solid nodules with a longest diameter of less than 10 mm that showed an increase in the longest diameter was quite limited. Further evaluation with larger patient and nodule numbers is needed to develop a follow-up system for solid nodules with more significant findings.
Conclusions
The 95% limits of agreement for volume changes in 5-year stable solid nodules may enable the detection of an increase or decrease in solid nodules at an earlier stage than that enabled by a clinical diagnosis, possibly contributing to the development of a follow-up system for reducing the number of additional CT scans performed during the follow-up period. Validation of our findings in a study with larger patient and nodule numbers is required.
Acknowledgements
Funding: This research was supported in part by a grant-in-aid from the Third-term Comprehensive Cancer Control Strategy sponsored by the Ministry of Health, Labour and Welfare, Tokyo, Japan (22-019), the National Cancer Center Research and Development Fund (27-A-5), Tokyo Japan, and a grant-in-aid from the Japan Agency for Medical Research and Development (AMED) for the Health and Labor Sciences Research Expenses for Commission (the Practical Research for Innovative Cancer Control: 15ck0106096h0002).
Footnote
Conflicts of Interest: Mr. Yamamichi is an employee of Canon Inc., Japan and was a visiting researcher of the Cancer Screening Division, Research Center for Cancer Prevention and Screening, National Cancer Center, and Dr. Oubel is an employee of MEDIAN Technologies, France. The other authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board in the National Cancer Center approved this study (approval number: 2012-345) and informed consent was obtained from all the patients.
References
- Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Xie X, Heuvelmans MA, van Ooijen PM, et al. A practical approach to radiological evaluation of CT lung cancer screening examinations. Cancer Imaging 2013;13:391-9. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48-56. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Kostis WJ, Yankelevitz DF, Reeves AP, et al. Small pulmonary nodules: reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology 2004;231:446-52. [Crossref] [PubMed]
- Revel MP, Lefort C, Bissery A, et al. Pulmonary nodules: preliminary experience with three-dimensional evaluation. Radiology 2004;231:459-66. [Crossref] [PubMed]
- Wormanns D, Kohl G, Klotz E, et al. Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol 2004;14:86-92. [Crossref] [PubMed]
- Goodman LR, Gulsun M, Washington L, et al. Inherent variability of CT lung nodule measurements in vivo using semiautomated volumetric measurements. AJR Am J Roentgenol 2006;186:989-94. [Crossref] [PubMed]
- Gietema HA, Wang Y, Xu D, et al. Pulmonary nodules detected at lung cancer screening: interobserver variability of semiautomated volume measurements. Radiology 2006;241:251-7. [Crossref] [PubMed]
- Gietema HA, Schaefer-Prokop CM, Mali WP, et al. Pulmonary nodules: interscan variability of semiautomated volume measurements with multisection CT-- influence of inspiration level, nodule size, and segmentation performance. Radiology 2007;245:888-94. [Crossref] [PubMed]
- Marchianò A, Calabrò E, Civelli E, et al. Pulmonary nodules: volume repeatability at multidetector CT lung cancer screening. Radiology 2009;251:919-25. [Crossref] [PubMed]
- Hein PA, Romano VC, Rogalla P, et al. Variability of semiautomated lung nodule volumetry on ultralow-dose CT: comparison with nodule volumetry on standard-dose CT. J Digit Imaging 2010;23:8-17. [Crossref] [PubMed]
- Ko JP, Berman EJ, Kaur M, et al. Pulmonary nodules: growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology 2012;262:662-71. [Crossref] [PubMed]
- Kim H, Park CM, Woo S, et al. Pure and part-solid pulmonary ground-glass nodules: measurement variability of volume and mass in nodules with a solid portion less than or equal to 5 mm. Radiology 2013;269:585-93. [Crossref] [PubMed]
- Smith GT, Rahman AR, Li M, et al. Reproducibility of volumetric computed tomography of stable small pulmonary nodules with implications on estimated growth rate and optimal scan interval. PLoS One 2015;10:e0138144. [Crossref] [PubMed]
- McNitt-Gray MF, Kim GH, Zhao B, et al. Determining the variability of lesion size measurement from CT patient data sets acquired under “No Change” conditions. Transl Oncol 2015;8:55-64. [Crossref] [PubMed]
- Sui X, Meinel FG, Song W, et al. Detection and size measurements of pulmonary nodules in ultra-low-dose CT with iterative reconstruction to low dose CT. Eur J Radiol 2016;85:564-70. [Crossref] [PubMed]
- Lung CT Screening Reporting and Data System (Lung-RADS™). Available online: https://www.acr.org/Quality-Safety/Resources/LungRADS
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Han D, Heuvelmans MA, Oudkerk M. Volume versus diameter assessment of small pulmonary nodules in CT lung cancer screening. Transl Lung Cancer Res 2017;6:52-61. [Crossref] [PubMed]
- Kakinuma R, Muramatsu Y, Kusumoto M, et al. Solitary pure ground-glass nodules 5 mm or smaller: frequency of growth. Radiology 2015;276:873-82. [Crossref] [PubMed]
- Sueoka-Aragane N, Kobayashi N, Bonnard E, et al. Evaluation of a cloud-based local-read paradigm for imaging evaluations in oncology clinical trials for lung cancer. Acta Radiol Open 2015;4:2058460115588103. [PubMed]
- Revel MP, Bissery A, Bienvenu M, et al. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable? Radiology 2004;231:453-8. [Crossref] [PubMed]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [Crossref] [PubMed]
- Sagawa M, Nakayama T, Tsukada H, et al. The efficacy of lung cancer screening conducted in 1990s: four case-control studies in Japan. Lung Cancer 2003;41:29-36. [Crossref] [PubMed]
- Suda K, Tomizawa K, Yatabe Y, et al. Lung cancers unrelated to smoking: characterized by single oncogene addiction? Int J Clin Oncol 2011;16:294-305. [Crossref] [PubMed]
- Heuvelmans MA, Oudkerk M, de Bock GH, et al. Optimisation of volume-doubling time cutoff for fast-growing lung nodules in CT lung cancer screening reduces false-positive referrals. Eur Radiol 2013;23:1836-45. [Crossref] [PubMed]
- Zhao YR, van Ooijen PM, Dorrius MD, et al. Comparison of three software systems for semi-automatic volumetry of pulmonary nodules on baseline and follow-up CT examinations. Acta Radiol 2014;55:691-8. [Crossref] [PubMed]
- Oda S, Awai K, Murao K, et al. Computer-aided volumetry of pulmonary nodules exhibiting ground-glass opacity at MDCT. AJR Am J Roentgenol 2010;194:398-406. [Crossref] [PubMed]


