Editorial on “Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size?”
The lung is a common site of metastases, originating from several extrapulmonary malignancies, including epithelial carcinomas and sarcomas. Prevalence depends on primary tumour site, which is generally colorectal cancer (CRC), breast cancer (BC) and genitourinary malignancies (1).
The presence of lung metastasis (LM) indicates an advanced disease (stage IV), with a 5-year survival rate of less than 5% for untreated patients (2).
The excision of pulmonary metastases has been initially applied in selected patients with single metastasis or long disease-free interval (DFI), with curative intent. Two decades ago, analyzing the International Registry of Lung Metastases (IRLM), Pastorino and Colleagues found 5,206 patients, who underwent pulmonary metastasectomy (PM) for LM from several malignancies (carcinomas, sarcomas, melanomas). The reported overall survival (OS) at 5 and 10 years was 36% and 26%, respectively (3). Similarly, Chudgar et al., reviewing a database of 539 patients undergoing PM for soft tissue sarcomas, reported a 5-year OS of 34% (4).
Based upon registry data and follow-up studies, and despite the absence of randomized trials, PM has become a well-established and accepted practice that reaches the 15% of all lung resections (5). However, no guidelines are available to define the resectability of LM from extrapulmonary malignancies, with the exception of CRC from the National Comprehensive Cancer Network (NCCN) (6). Although only retrospective and observational studies are so far available, most surgeons would accept some general criteria for resection of LM, as summarized in Table 1 (7,8). These criteria are similar to those originally described 60 years ago by Ehrenhaft, extended by recent technical advances (9). Anyway, the most important and widely accepted requisite is the completeness of resection, which is associated with better outcome. Pastorino et al. reported a 5-year survival rate of 36% for patients, in whom a complete resection was achieved, compared to 13% in case of incomplete resections (3).
The role of surgical margin has been studied in several malignancies, to find a uniform criterion, with the intent of reducing recurrence, improving patient survival, and preserving organ function. The latter is a crucial issue, particularly for the lung parenchyma, when multiple resections are needed or in case of repeated metastasectomies for relapse. Pulmonary resections inevitably affect lung function, and adjuvant therapies may further deteriorate it. No significant changes in quality of life (QoL) have been found after metastasectomies, even if deterioration in physical function after 3–6 months has been reported (10). Thus, an optimal management of surgical resection, and a balance between safety margins and parenchymal preservation are crucial in such patients.
Several studies on primary lung tumours confirmed that increasing the resection margin distance, in case of a sub-lobar resection for an early non-small cell lung cancer (NSCLC), a significant decrease of the risk of local recurrence can be achieved (11). Very recently, Wolf et al. reported also longer OS in case of margin distance greater than 11 mm, after wedge resection for stage I NSCLC (12). Differently from early-stage NSCLC, in metastatic disease a wedge resection is preferable, particularly for peripheral lesions, and to preserve parenchyma in case further metastasectomies are needed. Rusch et al. suggested a margin of 0.5–1 cm in all directions, in case of wedge resection or enucleation of the metastasis (13). A lobectomy may be reasonable also for LM, in case of hilar metastases or when multiple lesions are located in the same lobe (2). However, the heterogeneity of patients with LM included in the studies makes the comparison of the results difficult.
Welter et al. proposed an intriguing investigation on the dilemma of achieving a safe surgical margin, ensuring at the same time an adequate parenchymal preservation (14). Based on the hypothesis that the size and the biology of the tumour determine its invasiveness and ultimately the risk of recurrence, their analysis has shown crucial differences in growth patterns between various histologies. To the best of our knowledge, this is the first study that relates growth patterns of different histologies and the need of adequate tumour-lung interface.
The analysis of 412 specimens, obtained from 183 patients with LM from different primitive malignancies (CRC, epithelial, sarcoma, renal cell and melanoma), revealed several growth patterns, variously associated in the different histologies. Main characteristics and frequencies of the microscopical findings are summarized in Table 2.
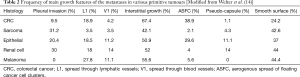
Full table
Kadota et al. found an aggressive pattern of lung adenocarcinoma, characterized by “spread through air spaces” (STAS), and associated with increased local recurrence after limited resections (15). An accurate analysis of the specimens is crucial, to avoid a false-negative diagnosis of free margin, if this specific pattern is not recognized by the pathologist. Currently, a margin distance lower than 1 cm and the presence of STAS are considered risk factors for local recurrence, in case of sub-lobar resections for early NSCLC (16). The same pattern was found also in metastases with smooth surface, from primitive renal cell cancer (RCC), previously considered a less aggressive characteristic of the metastasis. Welter and Colleagues found a similar pattern of tumour diffusion, called aerogenous spread of floating cancer cell clusters (ASFC), frequently associated with CRC metastases (38.9%). Interestingly, this pattern and tumour cells were present up to 7 mm from the metastasis, and confirmed the aggressive characteristic of this microscopical feature (14). The novelty of the study consists in the correlation between the growth pattern of the metastases and the safety margin of resection. In their series, Welter et al. found that 58% of LM had at least one pattern of aggressive diffusion, which needs an adequate distance between the nodule and the margin of resection. Thus, for metastases from CRC and epithelial tumours, which were found to exhibit ASFC, a margin greater than 7 mm is recommended. Furthermore, a linear correlation between the size of the lesion and the risk of aggressive growth pattern was found, confirming the need of a wider surgical margin for larger LM (14).
Differently, sarcoma metastases were found to exhibit “pleural infiltration” (infiltration of all visceral pleural layers), which increased the risk of local recurrence, together with a size of the lesion greater than 5 mm (17). Similarly, the presence of “Interstitial growth” pattern, defined as a diffuse spread of the sarcoma cells into the alveolar septae, was found to affect OS (18). The incidence of lymphangitic spread is very low in sarcoma metastases, and the presence of pseudo-capsule is rare (14,17). Sarcomas frequently and often exclusively metastatize to the lung. Thus, PM is an effective treatment of patients with sarcomas, and also patients with multiple lesions and repeated metastasectomies exhibited significant survival rates (17). Chudgar et al. reported a median OS of 33.2 months after PM, with 34% of patients alive at 5-years, whereas other Authors found 5-year survival rates up to 44.7% (4,18). Consequently, broad lateral margins and non-anatomical resections are recommended for LM from sarcoma (14).
The metastases from RCC were frequently associated with all aggressive growth patterns, and larger anatomical resection, together with intrapulmonary lymph nodes, may be needed (14). Hofmann et al. reported a median survival of 39.2 months, and a 5-years survival rate of 33.4% (19). Metachronous disease with long DFI, small size and up to 6 metastases are reported as good prognostic factors for PM with curative intent (19,20).
Melanoma nodules typically grow around vessels in the lung, but the aggressive behaviour and the tendency of diffusion to other sites explain the reported poor survival (14,21). A 5-years survival rate of 4% was found in 945 patients with LM from melanoma, whereas data from IRLM showed a median survival of 17 months, and an OS of 18% at 5 years (3,22). Furthermore, reported OS was 22% at 5 years and 16% at 10 years, if a complete resection was achieved, whereas no patient with incomplete resection was alive at 5 years. Similar to other histologies, patients with a DFI greater than 36 months and a reduced number of metastases showed a better OS. Four or more metastases were associated with a 5-year survival rate of 8% (21). Notably, all these findings were obtained prior to the use of the new immune checkpoint inhibitors for metastatic melanoma.
Several retrospective studies focused on the role of surgery in LM originating also from other histologies, and reported similar indications and prognostic factors.
The technique of resection of the metastases was found to influence the distance of the surgical margin. Resection with staples was preferred for anatomical resections, and showed the greater distance between tumour and surgical line (6.3±5.8 mm). Laser resection was related with the minor distance from the tumour (1.7±1.7 mm), and was used for multiple lesions and when parenchymal preservation was mandatory. However, no correlation between the type of device and the rate of local recurrence was found (14).
Although the heterogeneity of selection criteria and differences in variables analyzed, completeness of resection is considered the prerequisite of metastasectomy (2,3). Furthermore, DFI, number of metastases, size of the largest metastasis, and lymph node involvement were commonly reported as prognostic factors (2). Some other characteristics, such as preoperative CEA for CRC and hormone receptor status in BC, are considered in specific primary tumours.
Undoubtedly, multiple factors affect the outcome of patients after PM, and the presence of one or more poor prognostic factors may influence, but not necessarily contraindicate PM (23). Furthermore, there is a growing interest on the biology of the tumour and on the patterns of diffusion of the metastatic cells.
We have previously shown that the site of the primitive cancer (colorectal vs. non-colorectal) does not affect prognosis and survival (1). This preliminary report may correlate with the findings of Welter et al., suggesting a crucial role for growth patterns at a molecular level, rather than at a macroscopical one. The prognosis may be related to the pattern of microscopic growth, characteristic of each single histologic subtype, instead of a rough distinction between CRC and non-CRC malignancies. Unfortunately, the analysis for some subtypes of histology, with low prevalence, is difficult and not definitive, as correctly and clearly stated by Welter and Colleagues.
A 5-year OS rate up to 40% has been reported for patients who underwent PM, but most of the studies are retrospective analysis and include a heterogeneous population with different histologies and findings (lymph node status and adjuvant therapies) (7). Furthermore, the results of these studies may be affected by a selection bias, because only patients with favourable characteristics are included, or even a lead time bias, due to an earlier detection and a factitious longer recorded survival (24). The results from the ongoing Pulmonary Metastasectomy in Colorectal Cancer Trial (PulMiCC), will clarify the natural history of LM and the role of surgery, at least in CRC (25). However, the pivotal study of Welter et al. should drive the future research on growth patterns in studies with an adequate sample size, and justify the surgical treatment of patients with LM, based on the biological nature of the individual tumour (24).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lumachi F, Mazza F, Del Conte A, et al. Short-term survival of patients with lung metastases from colorectal and non-colorectal cancer who underwent pulmonary metastasectomy. Anticancer Res 2015;35:3563-6. [PubMed]
- Kim HK, Cho JH, Lee HY, et al. Pulmonary metastasectomy for colorectal cancer: how many nodules, how many times? World J Gastroenterol 2014;20:6133-45. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friendel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Chudgar NP, Brennan MF, Tan KS, et al. Is repeat pulmonary metastasectomy indicated for soft tissue sarcoma? Ann Thorac Surg 2017;104:1837-45. [Crossref] [PubMed]
- Van Raemdonck D. Pulmonary metastasectomy: common practice but is it also best practice? Future Oncol 2015;11:11-4. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN practice guidelines in oncology. Available online (accessed on November 20, 2017).http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Kondo H, Okumura T, Ohde Y, et al. Surgical treatment for metastatic malignancies. Pulmonary metastasis: indications and outcomes. Int J Clin Oncol 2005;10:81-5. [Crossref] [PubMed]
- Erhunmwunsee L, Tong BC. Preoperative evaluation and indications for pulmonary metastasectomy. Thorac Surg Clin 2016;26:7-12. [Crossref] [PubMed]
- Ehrenhaft JL, Lawrence MS, Sensenig DM. Pulmonary resection for metastatic lesions. AMA Arch Surg 1958;77:606-12. [Crossref] [PubMed]
- Welter S, Schwan A, Cheufou D, et al. Short- and mid-term changes in lung function after bilateral pulmonary metastasectomy. Ann Thorac Surg 2013;95:1006-11. [Crossref] [PubMed]
- Mohiuddin K, Haneuse S, Sofer T, et al. Relationship between margin distance and local recurrence among patients undergoing wedge resection for small (≤2 cm) non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:1169-75. [Crossref] [PubMed]
- Wolf AS, Swanson SI, Yip R, et al. The impact of margins on outcomes after wedge resection for stage I non-small cell lung cancer. Ann Thorac Surg 2017;104:1171-8. [Crossref] [PubMed]
- Rusch VW. Pulmonary metastasectomy. Current indications. Chest 1995;107:322S-31S. [Crossref] [PubMed]
- Welter S, Arfanis E, Cristoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences following limited resection for small stage I lung adenocarcinoma. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic impact of margin distance and tumor spread through air spaces in limited resection for primary lung cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Gafencu DA, Welter S, Cheufou DH, et al. Pulmonary metastasectomy for sarcoma – Essen experience. J Thorac Dis 2017;9:S1278-81. [Crossref] [PubMed]
- Welter S, Grabellus F, Bauer S, et al. Growth patterns of lung metastases from sarcoma: prognostic and surgical implications from histology. Interact Cardiovasc Thorac Surg 2012;15:612-7. [Crossref] [PubMed]
- Hofmann HS, Neef H, Krohe K, et al. Prognostica factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol 2005;48:77-81. [Crossref] [PubMed]
- Murthy SC, Kim K, Rice TW, et al. Can we predict long-term survival after pulmonary metastasectomy for renal cell carcinoma? Ann Thorac Surg 2005;79:996-1003. [Crossref] [PubMed]
- Leo F, Cagini L, Rocmans P, et al. Lung metastases from melanoma: when is surgical treatment warranted? Br J Cancer 2000;83:569-72. [Crossref] [PubMed]
- Harpole DH Jr, Johnson CM, Wolfe WG, et al. Analysis of 945 cases of pulmonary metastatic melanoma. J Thorac Cardiovasc Surg 1992;103:743-8. [PubMed]
- Quiros RM, Scott WJ. Surgical treatment of metastatic disease to the lung. Semin Oncol 2008;35:134-46. [Crossref] [PubMed]
- Åberg T, Treasure T. Analysis of pulmonary metastasis as an indication for operation: an evidence-based approach. Eur J Cardiothorac Surg 2016;50:792-8. [Crossref] [PubMed]
- Treasure T, Fallowfield L, Lees B, et al. Pulmonary metastasectomy in colorectal cancer: the PulMiCC Trial. Thorax 2012;67:185-7. [Crossref] [PubMed]



