Interrupted right-sided aortic arch: performance of umbilical xenograft after primary neonatal corrective surgery
Introduction
Interrupted aortic arch (IAA) is a rare cardiac malformation accounting for 1.5% of congenital cardiac anomalies (1). It is classified into three subtypes based on the level of interruption of the aortic arch (2). Type B represents the most common type with approximately 80% of the cases (3) with interruption occurring between subclavian and left carotid arteries. IAA is often accompanied by further cardiac malformations such as ventricular septal defect (VSD) or interruption of the pulmonary artery. Further, it can be associated with genetic disorders, such as DiGeorge syndrome (4). If untreated, this condition remains lethal usually 4–10 days after birth (4). The current therapy for IAA constitutes establishing ductal patency by infusing prostaglandin E1 followed by a one-stage primary neonatal repair including establishing arch continuation via direct anastomosis or tube graft and, if present, VSD closure. An obstruction of the graft is a common complication after the repair surgery with 45% occurrence within 5 years after the surgery (3). Other not uncommon complications include residual VSD and left ventricular outflow tract (LVOT) obstruction especially subaortic stenosis which influence early and late survival (5).
We present a follow-up case of a female patient with the history of IAA type B associated with right-sided descending aorta. She underwent corrective surgery as a neonate where a bovine umbilical vascular graft was used for aortic arch repair. Thirty-nine years later she is presenting with a dilatation of the xenograft and an aneurysm distal to it requiring a surgery. The initial case report describing the primary surgery was published by Wyler et al. in 1980 (6).
Case presentation
A 39-year-old female presented with aneurysm of distal aortic arch after undergoing corrective surgery for IAA as a neonate. She was diagnosed with type B IAA with concomitant VSD at birth. At the age of four days corrective surgery was performed using a bovine umbilical graft of 8 mm in diameter to restore the aortic arch, followed by ligation of ductus Botalli and banding of pulmonal artery via right thoracotomy. The VSD closure was performed four years later. Other than slight systolic pressure difference between the right and the left arm, the patient remained asymptomatic over the years. The clinical assessment and echocardiography were performed during regular check-ups at the department for congenital cardiac diseases. At the admission the patient did not show any symptoms related to the aortic arch aneurysm such as hoarseness of voice, shortness of breath, trouble swallowing or pain radiating to the back. The measurement of blood pressure showed an increase in systolic pressure difference currently of 30 mmHg between the right and the left arm. Due to the limited visualization of a possible aneurysm, an MR angiography was performed. A residual VSD or LVOT could be excluded via echocardiography.
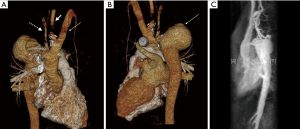
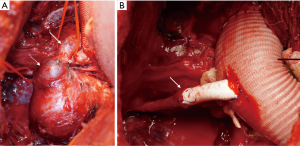
The magnetic resonance (MR) angiography showed the right-sided aortic arch with an aneurysm distal to the graft measuring 4.7×6.4×5.6 cm3 and a dilatation of the xenograft (Figure 1). Due to the significant caliber difference between the graft and aorta, making a stent-graft implantation unfeasible and due to the increased risk for aneurysm rupture a corrective surgery was preferred. The surgery was performed via Hemi-Clamshell-incision, since the location of the aneurysm limited sufficient access. The aneurysm and the graft-aorta anastomosis were exposed. The aorta ascendens continued into a common trunk, which divided into right common carotid artery and left-sided brachiocephalic artery (Figure 2A). The right subclavian artery originated dorsally to the aneurysm. A cardio-pulmonary bypass was established and the aorta was cross-clamped. The proximal graft-aorta anastomosis was exposed. A 26 mm Gelweave Dacron-prosthesis was used to replace the xenograft. The proximal end-to-side anastomosis was performed under deep hypothermic circulatory arrest. Next, the aneurysm was divided and removed. The right subclavian artery was dissected out and clamped. The distal anastomosis was performed. Due to the insufficient length of the subclavian artery, a polytetrafluoroethylene (PTFE)-prosthesis was used to connect the subclavian artery to the Dacron-prosthesis (Figure 2B). Finally, the aneurysm-wall was closed over the prosthesis. Postoperatively, the patient was transferred to the intensive care unit (ICU) and discharged after 10 days.
Discussion
An IAA with associated right-sided descending aorta is a rare condition. The current surgical treatment for IAA constitutes one-stage repair surgery during neonatal stage. The direct arch anastomosis or usage of Dacron-prosthesis and VSD closure represent the preferred approach. Our patient underwent a two-stage surgery, initially with establishing aortic continuity via a bovine umbilical graft and after four years VSD closure was performed. The initial surgical report regarding the surgery conducted at birth, published by Wyler et al. in 1980 (6), describes the preference for the biological heterograft because of its elasticity and easier handling. Moreover, the large distance between ascending and descending aorta prevented direct anastomosis at that time. The choice of a graft material is an important decision since this affects the further course of patient’s clinical outcome, quality of life, mortality and survival. There is no known literature about the course of material behavior of a bovine umbilical xenograft in a human over such a long time as 39 years. As for the vascular xenograft patency, there are some small-scale studies using bovine artery xenografts for hemodialysis access as an alternative to polytetrafluoroethylene (7). However, there is no clear evidence of its superiority. There is a need for large-scale studies in order to prove xenograft patency over the current available grafts. This case represents a unique insight into the long-term performance of an umbilical vascular xenograft with its declined patency over the time leading to graft dilatation and subsequent aneurysm.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Davis JA, Gilani R, Al-Najjar R, et al. Operative challenges in management of concurrent interrupted aortic arch and descending thoracic aortic aneurysm. J Vasc Surg 2013;57:1661-3. [Crossref] [PubMed]
- Celoria GC, Patton RB. Congenital absence of the aortic arch. Am Heart J 1959;58:407-13. [Crossref] [PubMed]
- Jonas RA. Management of Interrupted Aortic Arch. Semin Thorac Cardiovasc Surg 2015;27:177-88. [Crossref] [PubMed]
- Brown JW, Ruzmetov M, Okada Y, et al. Outcomes in patients with interrupted aortic arch and associated anomalies: a 20-year experience. Eur J Cardiothorac Surg 2006;29:666-73; discussion 673-4. [Crossref] [PubMed]
- Mishra PK. Management strategies for interrupted aortic arch with associated anomalies. Eur J Cardiothorac Surg 2009;35:569-76. [Crossref] [PubMed]
- Wyler F, Gradel E, Rutishauser M, et al. Successful palliation by means of a bovine artery graft in a 4-day-old infant with type B interruption of aortic arch and right descending aorta. Thorac Cardiovasc Surg 1980;28:57-60. [Crossref] [PubMed]
- Pineda DM, Dougherty MJ, Wismer MC, et al. Bovine carotid artery xenografts for hemodialysis access. J Vasc Surg 2017;65:1729-34. [Crossref] [PubMed]


