Peri-operative treatment of sleep-disordered breathing and outcomes in bariatric patients
Introduction
The rate of obesity in the UK is one of the highest in the world (1). As of 2013, 24% of men and 25% of women in the UK are classified as obese, with 3% classified as type III obese [previously known as “morbid obesity” with a body mass index (BMI) above 40 kg/m2] (2). Obesity is linked to several respiratory conditions such as pneumonia, asthma, pulmonary embolism and obstructive sleep apnoea (OSA) (3). However, despite the increasing prevalence of obesity, up to 90% of OSA remains undiagnosed (4): 4% of middle-aged men and 2% of middle-aged women in the UK suffer from sleep apnoea (5), but severe OSA occurs in 10–20% of patients with a BMI above 35 kg/m2 (6). OSA and obesity are significant contributors to cardiovascular and metabolic morbidity and mortality, associated with diabetes mellitus, ischaemic heart disease, hypertension and depression (5,6).
In OSA, transient hypoxias induced by upper airway occlusion in sleep activate the sympathetic nervous system, increase intra-thoracic pressure swings and cause arousal from sleep (5). Heart rate increases and sleep is fragmented, the sleep quality decreases (7), causing excessive daytime sleepiness, as well as impaired cognitive function, eventually leading to an impaired quality of life (5). OSA is diagnosed by experts with the help of a sleep study (polysomnography) in conjunction with a subjective increase in daytime sleepiness, assessed with the aid of an Epworth Sleepiness Scale (ESS) questionnaire (5).
Continuous positive airway pressure (CPAP) is currently the best available treatment for moderate to severe OSA, effective to avoid upper airway collapse, inflate the chest and diminish work for breathing and neural respiratory drive (5,8). In the context of surgery and anaesthetics, obesity and OSA are associated with a greater incidence in complications both peri- and post-operatively, leading to longer in-hospital stays and putting additional strain on healthcare providers (9). Obesity itself is not necessarily linked to increased risk of pulmonary complications pre- or post-operatively, even in the morbidly obese (10), however, in conjunction with OSA this is different (11). Patients with OSA are more than twice as likely to develop post-operative desaturations, cardiac complications, adult respiratory distress syndrome (ARDS), respiratory failure, aspiration pneumonia and other problems that require admission to intensive care (9,11). Frequent and extended desaturations also predispose patients towards opioid-induced respiratory depression due to hypoxaemia and hypercapnia (6). However, the risk of these complications is potentially reduced if OSA is identified pre-operatively and treated with CPAP (4,6,9,11).
We hypothesize that long-term and peri-operative complication rates are low in bariatric patients who are screened for OSA and, if required, treated accordingly. For this purpose, we identified peri- and post-operative mortality with long-term follow-up, as well as complication rate and length of hospital stay in all screened bariatric patients and compared the occurrence of complications between patients with moderate-severe OSA with those who had mild OSA or no sleep-disordered breathing.
Methods
Retrospective data of patients referred to the Lane Fox Unit (respiratory high dependency unit, St. Thomas’ Hospital, London, UK) and the Sleep Disorders Centre (Guy’s Hospital, London, UK) for assessment of sleep-disordered breathing in conjunction with bariatric surgery were analysed. The study was registered as a service review and approved by the local clinical governance board (registration number: GSTT-2017-6952). Cases were included between 12 June 2014 and 9 March 2017.
Variables recorded included age (years), gender (male and female), height (m), weight at time of sleep study (kg), BMI (kg/m2), neck circumference, waist circumference, comorbidities, medication, surgery date, surgery type, intra-operative complications, post-operative complications, length of post-operative hospital stay (days), ward vs. ITU stay, post-operative weight at last follow-up (kg), post-operative BMI at last follow-up (kg/m2), weight loss over the follow-up period (kg/year); it was recorded whether patients were prescribed CPAP, the respective CPAP pressure (in cmH2O), annual CPAP compliance (% of nights used), average nightly CPAP use (in hours), ESS (points) and Hospital Anxiety and Depression Scale (HADS, points), as well as the results of the nocturnal pulse oximetry data.
Questionnaires
Patients scheduled to undergo bariatric surgery at Guy’s and St. Thomas’ NHS Foundation Trust, London, UK were screened pre-operatively with the STOP-Bang questionnaire. STOP-Bang is an acronym for snoring (significantly loud), tired (excessive daytime sleepiness), observed apnoea (others seeing the patient choking during sleep), (blood) pressure (current or treated hypertension), BMI (>35 kg/m2), age (>50 years), neck circumference (>40 cm) and gender (male). Each parameter is graded “0” (for “no” answers) and “1” (for “yes” answers) (12). A score >4 represented a risk of OSA and indicated referral to the Sleep Disorders Centre for more extensive investigation.
The ESS was recorded; it asks patients to rate the probability of falling asleep during eight activities of daily living on a scale of “0” to “3”, with higher scores (>10) indicating daytime sleepiness (13).
The HADS is a scoring tool to assess patient’s levels of anxiety and depression. There are 14 statements to score from “0” to “3”, 7 about anxiety and 7 about depression. The questions deliberately avoid asking about sleep because changes in sleep may be symptoms of these disorders (14). This scale can be used in conjunction with the ESS (10). A score <9/21 on either questionnaire or a total score <18/42 points is deemed normal.
Nocturnal pulse oximetry
Patients recorded two nights of pulse oximetry at home using a Pulsox 300i (Konica Minolta sensing Inc., Hachioji, Tokyo, Japan). The data recorded and calculated included:
- Mean pulse rate (min−1);
- Number of pulse rises >six beats per minute (“pulse rises”) (h−1);
- Mean peripheral capillary oxygen saturation (SpO2, %);
- Nadir peripheral capillary oxygen saturation (SpO2, %);
- 4% and 3% oxygen desaturation index (ODI; h−1);
- Time spent with SpO2 <90% (% of total time recorded).
The results were further assessed by an expert sleep technician and scored by a consultant. OSA was defined by the number of desaturations a patient experienced per hour of sleep; the 4% ODI. A 4% ODI <5 events/hour was considered normal; 5–15 events/hour was mild, 15–30 events events/hour was moderate and >30 events/hour was considered severe OSA (9). Possible coexisting obesity hypoventilation syndrome (OHS) was indicated by prolonged periods (>10% of the recorded time) of hypoxia (SpO2 <90%) during sleep. Patients were assigned a value from 1–5 for the severity of their OSA:
- “1”—if no OSA present;
- “2”—if mild OSA present;
- “3”—if moderate OSA present;
- “4”—if severe OSA present;
- “5”—if patients had OSA, potentially combined with OHS.
Recommended treatments were assigned a value from 1–5:
- “1”—no further action necessary;
- “2”—recommendation of a mandibular advancement device if symptomatic;
- “3”—recommended extubation onto CPAP;
- “4”—peri-operative CPAP;
- “5”—referral for inpatient assessment to investigate the potential presence of OHS.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, IBM, Chicago, IL, USA) version 24.0. Categorical variables are summarised using percentages. A Shapiro-Wilk with Lilliefors significance correction test was used to determine whether continuous variables were normally or non-normally distributed. Normally-distributed continuous variables are cited as “mean (standard deviation)” and compared using an unpaired t-test. Non-normally distributed continuous variables are cited as “median [interquartile range (IQR)]” and compared using a Mann-Whitney test. Categorical variables were compared with a Chi-square test. A Pearson correlation test was used to compare independent and dependent variables; a Spearman correlation test was used to compare non-normally distributed data. A P value of <0.05 was deemed statistically significant.
Results
Demographics
A total of 410 patients awaiting bariatric surgery were studied, the majority were female, middle aged and obese [74% female (302:108), age 46.9 (11.9) years, weight 134.5 (27.5) kg, BMI: 48.1 (8.2) kg/m2, waist circumference of 136.7 (16.7) cm, neck circumference 43.6 (4.5) cm]. Four patients were excluded from the analysis because they had insufficient or unobtainable sleep results. The average of patients was not excessively sleepy, but were considerably susceptible to depression and/or anxiety [ESS 8.0 (5.0) points, total HADS 17.7 (7.3) points]. Most patients (85.9%, n=352) had a BMI >40 kg/m2, 12.4% (n=51) had a BMI between 35–40 kg/m2 and 1.7% (n=7) patients had a BMI between 30–35; 34.2% (n=140) of the patients were “super obese” with a BMI ≥50 kg/m2; the highest BMI was 88.9 kg/m2.
Sleep disordered breathing (SDB)
Nocturnal pulse oximetry data revealed mild-moderate OSA for the total cohort [4% ODI of 15.4 (17.3) h−1, 3% ODI of 20.1 (19.6) h−1, mean SpO2 of 94.1 (3.6) %, a nadir SpO2 of 75.9 (10.0) %, mean pulse rise index of 34.0 (18.6) h−1, and a time below SpO2 of 90% of 8.0 (14.2) % of the sleep study)]; 285 out of 410 patients (70%) had at least some degree of OSA, while 125 (30%) had no significant degree of OSA. Of those with any degree of OSA, 127 (31% of total patients) were mild, 82 (20% of total patients) were moderate, 57 (14% of total patients) had severe OSA and 19 (5%) had OSA with potentially combined OHS. Average follow-up time post-pulse oximetry was 432.9 (241.9) days.
Male vs. female groups
When comparing male with female patients, the male group was slightly older but of similar BMI, both groups had a similar sleep and mental health symptom score (ESS, HADS) and heart rate; there were about three times as many women as men in this cohort. There was no significant difference in length of hospital stay, with all but two patients admitted to a ward post-operatively; the two patients admitted to ICU were female. Men were on average heavier and had more severe sleep-disordered breathing, as indicated by the mean/minimum SpO2, the ODI and the percentage of the night with an SpO2 <90%, as well as the pulse rise index as an indicator of arousals; men were 20.3% more likely (85% of male patients compared to 64.7% of female patients) to require treatment recommendation like MAD, CPAP or referral for further respiratory assessment (Table 1).

Full table
Co-morbidities
Hypertension was present in 42% (n=171) patients, 37% (n=153) patients had diabetes mellitus, 25% (n=104) had depression, 9% (n=37) had asthma, 7% (n=30) had high cholesterol/dyslipidaemia, 7% (n=28) had polycystic ovarian syndrome (PCOS), 5% (n=19) had impaired glucose tolerance, 5% (n=19) had anxiety, 3% (n=13) had chronic obstructive pulmonary disease (COPD), 2% (n=9) had chronic kidney disease (CKD)/renal impairment, 2% (n=8) had epilepsy or a history of seizures.
Medication
Twenty-five percent (n=102) were taking one (or more) oral hyperglycaemic agent(s) (metformin, sitagliptin, gliclazide, liraglutide), 21% (n=87) were taking a statin (simvastatin, atorvastatin, rosuvastatin), 17% (n=69) were taking one (or more) antidepressant(s) (escitalopram/citalopram, fluoxetine/duloxetine/paroxetine/sertraline, amitriptyline), 17% (n=68) were taking a proton-pump inhibitor (omeprazole, lansoprazole), 16% (n=64) were taking a calcium channel blocker (amlodipine, nifedipine, diltiazem), 15% (n=61) were taking an angiotensin-converting enzyme inhibitor (“ACEi”; ramipril, lisinopril, perindopril, enalapril), 14% (n=59) were taking one (or more) inhaler(s) (salbutamol, salmeterol, seretide, ventolin, tiotropium, symbicort, clenil modulite, beclomethasone, fluticasone), 9% (n=37) were taking one (or more) injected insulin(s) (Humulin, Humalog, “insulin”, novomix, Novorapid), 9% (n=37) were taking an angiotensin II receptor blocker (“ARB”; losartan, irbesartan), 6% (n=25) were taking one (or more) anti-convulsant (gabapentin, pregabalin, carbamazepine, valproate), 6% (n=25) were taking orlistat.
CPAP treatment
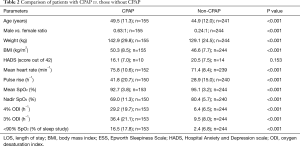
A total of 220 (54%) patients received no further treatment, 164 (40%) patients were prescribed CPAP treatment and 26 (6%) patients were recommended a mandibular advancement device for their symptoms. Patients who required CPAP therapy were, on average, older, heavier, with a higher BMI and more symptomatic than patients without, as via selection, they had a more severe degree of OSA (Table 2). Of those on CPAP (n=164), 125 (76%) were prescribed CPAP pre- and post-operatively, 17 (10%) were prescribed post-operative extubation onto CPAP, and 22 (13%) were referred to the Lane Fox Unit for further respiratory assessment of non-invasive ventilation (NIV) vs. CPAP; 2 of the patients referred for further assessment did not require CPAP. The patients with CPAP had an average pressure of 13.7 (2; n=81) cmH2O, an annual compliance of 64.0% (34.0; n=50), and nightly use of 4.5 (1.9; n=50) hours. The length of stay in hospital for the operated cohort was similar between CPAP and non-CPAP group.
Full table
Bariatric surgery
During the follow-up period (i.e., days since sleep study recording) of 719.4 (143; n=53) days 53/413 (12.8%) patients had undergone bariatric surgery [80% female (44:9), age 48.5 (11.4) years, 138.0 (28.5) kg, 49.3 (8.2) kg/m2]. 48 patients were class III obese, including 20 patients who were “super obese”, and 5 patients were class I and II obese. 47% (n=25) patients had hypertension, 30% (n=16) had type 2 diabetes mellitus, 28% (n=15) patients had depression, 11% (n=6) had COPD, 9% (n=5) had asthma. The patients had undergone either a laparoscopic sleeve gastrectomy (SG; n=31), a Roux-en-Y gastric bypass (RYGB; n=20), and in two patients the type of surgery was not documented, with or without a simultaneous hernia repair. There were no patient deaths in the operated population during the data collection period.
There were no significant differences between the surgical and non-surgical population with regards to demographics, symptom scores or nocturnal pulse oximetry data. CPAP treatment had been commenced in 47% (25:28) of this cohort, mean CPAP pressure was 13.1 (2.2) cmH2O, nightly CPAP use was 4.2 (2.1) hours, and the ESS was 7.8 (5.0) points on treatment. The mean post-operative follow-up time was 375.3 (277.9) days. By then, patients had an average weight loss of 22.0% (10.5) of their body weight with no significant difference between the groups (Table 3). The average length of stay in hospital was 2.5 (1.2) days, there was no difference between patients on CPAP compared to those without [CPAP 2.6 (1.4) vs. no CPAP 2.4 (1.0) days, P=0.650].

Full table
Out of the 53 surgical patients, only 2 had any pre-, intra- or post-operative complications:
- A 63-year-old female patient with COPD, not receiving CPAP, suffered from an acute kidney injury (AKI) post laparoscopic sleeve gastrectomy. This patient was treated on a non-ICU hospital ward for 2 days before being discharged; the patient has not had any relevant medical complications in the 9 months since surgery;
- A 62-year-old female patient with COPD, extubated onto CPAP, suffered from hypercapnic (type 2) respiratory failure (HCRF) post-laparoscopic sleeve gastrectomy. This patient was admitted to ICU, and was treated in ICU for 2 days before being discharged. This patient has not suffered any relevant medical complications in the 4 months since surgery.
Discussion
OSA is the most common form of SDB and possibly the most prevalent chronic respiratory disorder (8). OSA is compounded by the fact that the morbidly obese population breathe at a reduced functional residual capacity, approaching the residual volume, which reduces lung compliance, increases the physical work of breathing and minimizes the pressure gradient between the alveolar and intrapleural pressure (trans-pulmonary pressure) (8) necessary to drive minute ventilation for continuous gas exchange (6,9). However, increased levels of neural respiratory drive in obesity decrease with the onset of sleep (8). In addition, basal metabolic rate and oxygen demand are increased, leading to a rapid decline in arterial oxygen saturation when breathing is interrupted (6). Increased intra-abdominal pressure creates intrinsic positive end-expiratory pressure, particularly in supine posture (8), and functions as a limit to the inspiratory load (6,9,15).
Summary
In a large cohort of bariatric surgery patients, sleep-disordered breathing is highly prevalent: 70% had some form of OSA, while 40% required peri-operative planning with CPAP therapy. Obese men had more severe OSA, while the majority of bariatric patients was female. Although only a minority of 13% had been operated during the follow-up period, only one patient (2% of the surgical cohort), who had COPD and was extubated onto CPAP, suffered post-operative respiratory complications. None of the other patients had any significant respiratory peri-operative complications. There was no significant difference in length of hospital stay between operated patients with CPAP compared to the non-CPAP cohort. Pre-operative established CPAP therapy and/or extubation onto CPAP proved to deliver the same outcomes as in the patient cohort with no or only mild OSA; CPAP therapy has proven to deliver an effective method of controlling respiratory complications during and after surgery.
Clinical significance
Previous studies have found similar results with regards to rates of OSA in bariatric surgery cohorts, between 73% and 78% (12,13,16) of bariatric surgery patients have some form of sleep-disordered breathing, with 24% suffering from moderate to severe OSA and 11% having findings suggestive of combined OSA/OHS (12).
Sleep-disordered breathing is a significant risk factor for perioperative complications: a systematic review identified eight studies linking OSA to higher rates of hypercapnic respiratory failure (17). Patients with moderate to severe sleep-disordered breathing are at higher risk of mortality, sepsis and respiratory complications after elective coronary artery bypass-graft surgery (18). Patients with OSA and unidentified OHS had higher risks of postoperative respiratory failure, heart failure, prolonged intubation, and longer ICU and hospital length of stay than patients with non-hypercapnic OSA (19).
Obese patients with OSA present a challenge to anaesthetists, with a significantly higher risk of difficult mask ventilation and laryngoscopy (20), as well as requiring more intubation attempts (21). Meta-analyses have established that patients with OSA are more than twice as likely to suffer peri-operative respiratory complications compared to patients without OSA (22,23). Many other types of surgery carry an inherently increased risk of respiratory complications, even in patients with no prior risk factors (11). Screening for OSA may thus be applicable to these types of surgery as well as bariatric surgery.
Besides a difficult airway with high Mallampati score, COPD is one of the most commonly identifiable causes of post-operative pulmonary complications. OSA may be prevalent in 13.8% of COPD patients and significantly compounds peri-operative risk (24). Pre-operative screening for OSA (and treatment with CPAP if necessary) in pre-surgical COPD patients may be of clinical importance. However, 23% of patients with combined COPD and OSA may require bilevel positive airway pressure (BiPAP) for associated hypoventilation with hypercapnic respiratory failure (24). Hypercapnic (type 2) respiratory failure increases mortality (25), particularly in patients with COPD, even in those treated on a respiratory ITU (26). The COPD patient who suffered from a post-operative HCRF in our cohort may therefore have required non-invasive ventilation instead of CPAP and as such may not represent a lack of efficacy in peri-operative CPAP treatment.
CPAP is a routine and beneficial treatment for moderate to severe OSA (27) and has been recorded as an effective method of reducing peri-operative respiratory complications compared to patients with undiagnosed OSA without receiving CPAP (11,28). Although CPAP does reduce symptoms relating to apnoea, such as abnormalities in night-time oxygen levels and excessive daytime sleepiness, it has also positive effects on memory and cognition (29,30), improves quality of life, reduces blood pressure (31) and improves cardiac outcomes (32). However, despite patients experiencing relief of sleep-related symptoms, compliance to CPAP treatment remains suboptimal, with as many as half of the patients not sufficiently adhering to treatment at 1 year (17). Indeed, our study found that patients only used their prescribed CPAP during two out of every three nights, on average.
The similar length of stay between the groups with and without OSA highlights the effectiveness of CPAP—patients prescribed appropriate CPAP had outcomes and hospital stays comparable to patients who did not require it at all. This demonstrates that CPAP is effective in managing the respiratory needs of bariatric patients and those with OSA.
The ESS is the most commonly used questionnaire in Sleep Med (13), but it does not differentiate between various causes of excessive daytime sleepiness (EDS) (33), or correlate with objective measures of EDS (9) and its applicability to obese patients is controversial (34). For these reasons, the STOP-Bang questionnaire has taken precedence for OSA screening in obese patients in the pre-operative clinics (20). The STOP-Bang questionnaire is based on snoring, daytime tiredness, observed apnoeic episodes, BMI over 35 kg/m2, advanced age, male gender, a history of hypertension and neck circumference (35). These criteria are highly sensitive (94–96%) in the diagnosis of moderate-severe OSA (36).
Limitations
Patients studied were selected solely based on high-risk STOP-Bang scores, thus patients with lower scores were not followed. Future research should attempt to include all surgical patients to determine the presence (or absence) of any peri-operative respiratory complications in those not screened via the sleep service. Additionally, although highly sensitive, the STOP-Bang score is not a measure of OSA, and patients with actual OSA may not have a high STOP-Bang score, excluding them from the cohort despite their suitability for treatment.
Furthermore, all patients with moderate-severe OSA were treated with CPAP, and we cannot claim that there was an increased risk of complications if these patients had remained untreated. However, the fact that overall risks were low and comparable between groups is reassuring in that the proposed management plan is sufficient. In the context of a randomised controlled clinical trial, the use of sham-CPAP, an effective placebo for CPAP (37), if ethically acceptable, would provide more evidence about the effectiveness of peri-operative CPAP. Hip circumference measurements would have been of interest for assessing insulin resistance. HAD scores for surgical patients would have also been useful to assess the psychological profile of this cohort. However, the surgical cohort matched the non-surgery cohort, and it is therefore likely that the HAD scores could be assumed to be similar. Additionally, although CPAP is effective in OSA, BiPAP may be more suitable for patients with combined OSA and COPD.
Lastly, this study focused on peri-operative outcomes in bariatric/abdominal surgery, but pre-operative OSA screening and subsequent CPAP treatment may be applicable to other forms of (respiratory) high-risk surgery as well.
Conclusions
Bariatric patients screened pre-operatively for OSA and treated with CPAP, according to guidelines, suffer few respiratory complications. Pre-operative screening for OSA and peri-operative treatment with CPAP in morbidly obese patients is an effective method of reducing respiratory disease-related morbidity and mortality in the bariatric population.
Acknowledgements
Dr. Steier’s contribution to this project was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London, who part funded his salary. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local clinical governance board (GSTT-2017-6952).
References
- McPherson K, Marsh T, Brown M. Tackling obesities: future choices—modelling future trends in obesity and the impact on health. UK Government Office for Science. 2nd ed. Available online: www.foresight.gov.uk
- Health and Social Care Information Centre. Statistics on Obesity, Physical Activity and Diet. Leeds, England: Health and Social Care Information Centre, 2013.
- Zammit C, Liddicoat H, Moonsie I, et al. Obesity and respiratory diseases. Int J Gen Med 2010;3:335-43. [PubMed]
- Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology 2014;121:707-18. [Crossref] [PubMed]
- Steier J, Martin A, Harris J, et al. Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision across the UK. Thorax 2014;69:390-2. [Crossref] [PubMed]
- Members of the Working Party, Nightingale CE, Margarson MP, et al. Peri-operative management of the obese surgical patient 2015: Association of Anaesthetists of Great Britain and Ireland Society for Obesity and Bariatric Anaesthesia. Anaesthesia 2015;70:859-76. [Crossref] [PubMed]
- Pengo MF, Drakatos P, Kosky C, et al. Nocturnal pulse rate and symptomatic response in patients with obstructive sleep apnoea treated with continuous positive airway pressure for one year. J Thorac Dis 2014;6:598-605. [PubMed]
- Steier J, Jolley CJ, Seymour J, et al. Neural respiratory drive in obesity. Thorax 2009;64:719-25. [Crossref] [PubMed]
- Steier J, Kent B. Assessment and treatment of sleep-disordered breathing in the morbidly obese surgical patient. Cambridge University Press, 2017. [Epub ahead of print].
- Smetana GW. Preoperative pulmonary evaluation. N Engl J Med 1999;340:937-44. [Crossref] [PubMed]
- Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med 2006;144:575-80. [Crossref] [PubMed]
- Reed K, Pengo MF, Steier J. Screening for sleep-disordered breathing in a bariatric population. J Thorac Dis 2016;8:268-75. [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [Crossref] [PubMed]
- Bjelland I, Dahl AA, Haug T, et al. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002;52:69-77. [Crossref] [PubMed]
- Steier J, Jolley CJ, Seymour J, et al. Increased load on the respiratory muscles in obstructive sleep apnea. Respir Physiol Neurobiol 2010;171:54-60. [Crossref] [PubMed]
- Lopez PP, Stefan B, Schulman CI, et al. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg 2008;74:834-8. [PubMed]
- Opperer M, Cozowicz C, Bugada D, et al. Does Obstructive Sleep Apnea Influence Perioperative Outcome? A Qualitative Systematic Review for the Society of Anesthesia and Sleep Med Task Force on Preoperative Preparation of Patients with Sleep-Disordered Breathing. Anesth Analg 2016;122:1321-34. [Crossref] [PubMed]
- Rupprecht S, Schultze T, Nachtmann A, et al. Impact of sleep disordered breathing on short-term post-operative outcome after elective coronary artery bypass graft surgery: a prospective observational study. Eur Respir J 2017;49:pii1601486. [Crossref] [PubMed]
- Chung F, Nagappa M, Singh M, et al. Contemporary Reviews in Sleep Med CPAP in the Perioperative Setting: Evidence of Support. Chest 2016;149:586-97. [Crossref] [PubMed]
- Kheterpal S, Healy D, Aziz MF, et al. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the multicenter perioperative outcomes group. Anesthesiology 2013;119:1360-9. [Crossref] [PubMed]
- Stierer TL, Wright C, George A, et al. Risk assessment of obstructive sleep apnea in a population of patients undergoing ambulatory surgery. J Clin Sleep Med 2010;6:467-72. [PubMed]
- Kaw R, Chung F, Pasupuleti V, et al. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth 2012;109:897-906. [Crossref] [PubMed]
- Hai F, Porhomayon J, Vermont L, et al. Postoperative complications in patients with obstructive sleep apnea: a meta-analysis. J Clin Anesth 2014;26:591-600. [Crossref] [PubMed]
- Kuklisova Z, Tkacova R, Joppa P, et al. Severity of nocturnal hypoxia and daytime hypercapnia predicts CPAP failure in patients with COPD and obstructive sleep apnea overlap syndrome. Sleep Med 2017;30:139-45. [Crossref] [PubMed]
- Hukins C, Wong M, Murphy M, et al. Management of hypoxaemic respiratory failure in a Respiratory High-dependency Unit. Intern Med J 2017;47:784-92. [Crossref] [PubMed]
- Khilnani GC, Banga A, Sharma SK. Predictors of mortality of patients with acute respiratory failure secondary to chronic obstructive pulmonary disease admitted to an intensive care unit: a one year study. BMC Pulm Med 2004;4:12. [Crossref] [PubMed]
- Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006.CD001106. [PubMed]
- Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005;352:1206-14. [Crossref] [PubMed]
- Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res Bull 2003;61:87-92. [Crossref] [PubMed]
- Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on Cognition, Brain Function, and Structure Among Elderly Patients With OSA: A Randomized Pilot Study. Chest 2015;148:1214-23. [Crossref] [PubMed]
- McNicholas WT, Bonsigore MR. Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 2007;29:156-78. [Crossref] [PubMed]
- Doherty LS, Kiely JL, Swan V, et al. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005;127:2076-84. [Crossref] [PubMed]
- Slater G, Steier J. Excessive daytime sleepiness in sleep disorders. J Thorac Dis 2012;4:608-16. [PubMed]
- Slater G, Pengo MF, Kosky C, et al. Obesity as an independent predictor of subjective excessive daytime sleepiness. Respir Med 2013;107:305-9. [Crossref] [PubMed]
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812-21. [Crossref] [PubMed]
- Nagappa M, Liao P, Wong J, et al. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0143697. [Crossref] [PubMed]
- Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep 2010;33:260-6. [Crossref] [PubMed]


