A pooled analysis of advanced nonsquamous non-small cell lung cancer patients with stable treated brain metastases in two phase II trials receiving bevacizumab and pemetrexed as second-line therapy
Introduction
Brain metastases are a common complication of advanced non-small cell lung cancer (NSCLC) both at initial presentation and at the time of disease progression, with an estimated cumulative incidence of over 25% (1). Though historically associated with very poor prognosis (2), increasingly sophisticated diagnostic and central nervous system (CNS)-directed therapeutic modalities have improved survival over time (3). Patients with brain metastases have often been excluded from large randomized phase III trials due to concerns of poorer survival and impaired ability of drugs to cross the blood-brain barrier (4). However, as survival has improved, some trials have included such patients, often finding similar benefit to patients with metastatic disease elsewhere. For example, in a large phase III three-arm trial comparing the doublet carboplatin and paclitaxel to two gemcitabine-based regimens, 17.1% of 1,135 enrolled patients had stable brain metastases, and there were no significant differences in response, survival or toxicity between patients with and without brain metastases (5).
Bevacizumab (Avastin; Genentech, Inc., South San Francisco, CA, USA), a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF), has emerged as an important adjunct to platinum-based chemotherapy doublets for use in advanced NSCLC based on a pivotal phase III trial showing an overall survival (OS) benefit of 2.3 months when added to first line chemotherapy (6). However, given concern about the theoretical risk of CNS hemorrhage when using a VEGF-directed agent, especially after a grade 3 intra-cranial hemorrhage (ICH) was noted in a patient with hepatocellular carcinoma-associated brain metastatic disease in the original phase I study (7), patients with brain metastases were excluded from the trial. ICH is a feared complication of brain metastases from the lung, but data suggest that incidence is fairly low. A retrospective review of patients at M. D. Anderson Cancer Center showed that among 776 patients with brain metastases and 1,367 without, though the rate of ICH was higher in those with brain metastases (1.17% vs. 0.30%, P=0.013), there was no significant difference when only symptomatic ICH was analyzed (8). Given this low incidence, and the concern that a large proportion of lung cancer patients may be denied efficacious treatment, there has been interest in studying the use of bevacizumab in patients with brain metastases.
The safety of bevacizumab in first- and second-line use in NSCLC patients with stable treated brain metastases was specifically evaluated in a phase II trial, AVF3752g (PASSPORT). The primary endpoint was incidence of grade 2 or higher CNS hemorrhage, and among 115 enrolled patients, no such events were noted (9). Though PASSPORT suggested the safety of bevacizumab in patients with stable treated brain metastases, efficacy was not reported from that study.
Pemetrexed (Alimta; Eli Lilly and Co., Indianapolis, IN, USA), a multi-targeted anti-folate agent, is approved for use in first-line (with platinum), maintenance, and second-line treatment of advanced nonsquamous NSCLC, and is notable for its favorable toxicity profile compared to other agents (10). Based on the efficacy of pemetrexed as a second line agent and the safety questions surrounding bevacizumab in those with treated brain metastases, a trial (here referred to as the Stanford trial) was designed to look at the combination of both agents as a second line therapy in NSCLC patients with treated stable brain metastases. Due to the selective nature of the trial, accrual was slow and after the safety data from PASSPORT became available, further accrual was halted. To focus further on the efficacy question, in this analysis we pooled data from PASSPORT with the Stanford trial to look specifically at both the safety and efficacy of bevacizumab and pemetrexed when used as second-line treatment in NSCLC patients with stable treated brain metastases.
Since the completion of the studies, safety and efficacy of bevacizumab in active brain metastases have been further studied. De Braganca et al. reported in a retrospective case series that bevacizumab may be safe and effective for progressive brain metastases from NSCLC (11). The BRAIN study, a non-randomized phase II study examining carboplatin/paclitaxel plus bevacizumab in patients with NSCLC and asymptomatic untreated brain metastases, showed encouraging efficacy and safety bevacizumab in the combination (12). Our data further add to confirm the safety and efficacy of the use of bevacizumab in patients with brain metastases.
Methods
Studies
We pooled results from two phase II trials. The Stanford trial (NCT00227019) was an investigator-initiated study that enrolled advanced NSCLC patients with stable treated brain metastases for second-line treatment with bevacizumab and pemetrexed. The PASSPORT trial (NCT00312728) was an industry-sponsored multi-institution study that enrolled NSCLC patients with stable treated brain metastases for first- and second-line treatment with a variety of regimens; second-line patients could be treated with bevacizumab and the investigator’s choice of pemetrexed, docetaxel, erlotinib or another chemotherapy regimen, but only those patients receiving bevacizumab and pemetrexed were included in this pooled analysis.
Patients
Enrollment criteria were similar between the two studies. Patients with advanced stage nonsquamous NSCLC and stable treated metastatic disease in the brain were eligible if they were candidates for second-line therapy after progression on a platinum doublet regimen for advanced disease. The definition of stable brain metastases differed slightly between the two protocols. The Stanford trial required 1 month of radiographic stability after radiation and/or neurosurgery without ongoing use of steroid treatment. The PASSPORT trial required three months of radiographic stability after surgery, but after radiation, pemetrexed could be started as soon as 1 week after completion, while bevacizumab could only be started after 4 weeks. Subjects on both studies had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate baseline hematologic, renal and hepatic function, including neutrophils ≥1,500/µL, platelets ≥100,000/µL, hemoglobin ≥9 (PASSPORT) or 10 g/dL (Stanford), creatinine ≤1.5 times the upper limit of normal, and AST and ALT ≤2.5 times the upper limit of normal (though up to 5 times was allowed on PASSPORT in the presence of liver metastases). Urine protein/creatinine ratio had to be less than 1.0. Patients were excluded for history of hemoptysis (defined as 0.5 teaspoon or more of red blood ever for the Stanford study and within 3 months for PASSPORT). Patients were also excluded for other factors that would make them ineligible for bevacizumab according to the criteria listed in its prescribing instructions, including uncontrolled hypertension over 150/100, unstable angina or New York Heart Association grade II or higher congestive heart disease, myocardial infarction or stroke within 6 months. Women and men of childbearing potential had to agree to use effective contraception for the duration of the study. All patients on both trials provided written informed consent as approved by local IRBs prior to any study related procedures.
Treatment and evaluation
Patients on both studies received pemetrexed 500 mg/m2 and bevacizumab 15 mg/kg every 3 weeks until disease progression, unacceptable toxicity, discontinuation from study, or death. Patients received an intramuscular injection of vitamin B12 1,000 µg 1–2 weeks before initiation of pemetrexed, and every 9 weeks subsequently. Patients also were required to take folic acid 350–1,000 µg daily at least 1 week prior to initiation, to be continued until 3 weeks beyond the last pemetrexed dose. Dexamethasone 4 mg twice daily was given the day before, the day of, and the day after pemetrexed infusion to reduce the risk of rash. Bevacizumab was infused at a rate of 90 minutes for the first dose, which could be reduced to 60 minutes for the second dose and then 30 minutes for the third and subsequent doses if there were no infusion reactions. Specific adverse events requiring discontinuation on both studies included nephrotic syndrome, any grade arterial thromboembolic event, wound dehiscence requiring medical or surgical intervention, and gastrointestinal perforation. Grade 4 hypertension compelled discontinuation from both studies; grade 3 hypertension not controlled on medication also required discontinuation from PASSPORT. With respect to hemorrhage, patients had to be discontinued for grade 4 hemorrhage on the Stanford study and for grade ≥2 pulmonary or symptomatic CNS hemorrhage, or any grade ≥3 hemorrhage, on PASSPORT.
Imaging with brain MRI and CT chest and, as applicable, abdomen and pelvis, was performed every two cycles. The Stanford study planned pauses in accrual for safety evaluation after two patients began treatment until their first radiographic evaluation at 6 weeks, and again after 10 patients. PASSPORT stipulated suspension of study in the event of: three or more grades ≥two CNS hemorrhage events before treatment of the 16th subject; four or more before treatment of the 31st subject; or a 5th event observed at any point.
Analysis
The primary endpoint of both trials related to safety, specifically the incidence of CNS hemorrhagic events in the Stanford trial and incidence of symptomatic National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) grade 2+ CNS hemorrhage in PASSPORT. Patients in both studies were evaluable for progression-free survival (PFS) and OS, though only the Stanford study collected best radiographic response. For our analysis, we summed the number of safety events noted in the two trials, including the primary endpoints (CNS hemorrhage) and all grade 3–5 CTCAE events. We used IBM SPSS Statistics version 19.0 (SPSS, Inc., Chicago, IL, USA) to generate Kaplan-Meier survival curves for PFS and OS from the pooled data. We also present the radiographic response data from the Stanford study alone.
Results
Patients
A total of 38 patients from the two studies were included in the analysis. On the Stanford trial, 16 patients were accrued at two sites between April 2006 and April 2009. PASSPORT was fully accrued with 115 patients at 44 centers enrolled between March 2006 and June 2008. Of these, 22 patients from 13 centers received bevacizumab and pemetrexed and were included in this analysis. Table 1 shows baseline characteristics of the study populations, including demographics, histology, and the nature of their treatment for brain metastases. Though roughly evenly matched for age and sex, the Stanford trial population had a substantial proportion of Asian/Pacific Islander patients (38%). The PASSPORT trial had a larger proportion of large cell carcinoma histology. Data for smoking status were not collected on PASSPORT, but 50% of patients on the Stanford trial were never smokers.

Full table
Safety
No CNS hemorrhage events were noted in either study population. Two grade 5 events were observed: acute renal failure (ARF) and respiratory distress. Three grade 4 events were noted: neutropenia, pneumonia and severe fatigue. Two grade 3 thromboembolic events requiring anticoagulation occurred, possibly related to bevacizumab, both on the Stanford study. All grade 3 through five adverse events are reported in Table 2.
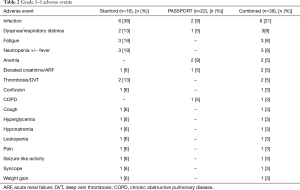
Full table
Efficacy
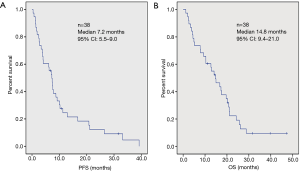
Kaplan-Meier curves for PFS and OS are shown in Figure 1. Median PFS was 7.2 months [95% confidence interval (CI): 5.5–9.0]. Median OS was 14.8 months (95% CI: 9.4–21.0). Best radiographic response was not a primary or secondary endpoint on PASSPORT, so data were not collected, but among the Stanford cohort, 4 (25%) had a partial response and 10 (63%) had stable disease.
Discussion
Brain metastases are a common presentation in advanced NSCLC, and traditionally were often treated with whole brain radiotherapy. Stereotactic radiosurgery (SRS) can be utilized when the number of brain metastases is limited (13), but for many patients with more extensive brain metastases, this is not an option. With improving prognosis for many patients with advanced NSCLC, the neurocognitive complication of whole brain radiotherapy is of increasing concern. Thus, the safety and efficacy of systemic therapies in treating brain metastases is an area under active investigation.
Patients with brain metastases were excluded from the registration trials of bevacizumab that showed a survival benefit with the use of angiogenesis inhibition. PASSPORT and the Stanford trial were both designed to evaluate the risk of CNS hemorrhage in patients with stable treated brain metastases, and PASSPORT’s favorable safety results have been presented previously (9). The current analysis adds further evidence of safety, with no CNS events noted among 38 total patients treated second-line with bevacizumab and pemetrexed.
As mentioned earlier, since the completion of the studies, safety and efficacy of bevacizumab in active brain metastases have been further studied. No CNS hemorrhage was noted in patients receiving either platinum-based chemotherapy with bevacizumab followed by bevacizumab with or without erlotinib on the phase III ATLAS trial (14,15), or oxaliplatin, pemetrexed and bevacizumab in a phase II trial (16). De Braganca et al. reported in a retrospective case series that bevacizumab may be safe and effective for progressive brain metastases from NSCLC (11). The BRAIN study, a non-randomized phase II study examining carboplatin/paclitaxel plus bevacizumab in patients with NSCLC and asymptomatic untreated brain metastases, showed encouraging efficacy and safety bevacizumab in the combination (12).
Indeed, given the role of VEGF in the growth of brain metastases (17) and in mediating the peritumoral edema that causes or aggravates many of the symptoms of metastases (18), future work should specifically include this population. Ongoing work in VEGF inhibition includes tyrosine kinase inhibitors; a phase II study of sunitinib, which targets VEGF receptors 1, 2 and 3 (among others), in a population of patients with treated brain metastases also showed no increased risk of ICH (19).
Another future research focus should be the appropriate combination of antiangiogenic agents with cytotoxic chemotherapy. A limitation of our study is a lack of data collected on radiographic response specifically of the CNS lesions after therapy. Though pre-clinical evidence suggests that distribution of pemetrexed to the brain is limited (20), there have been reports of efficacy against CNS lesions with use of pemetrexed alone, including one retrospective review of 39 patients showing a remarkable 82% CNS radiological benefit (21,22). Overall efficacy and disease control rate in our study, however, are quite encouraging compared to those found in previous studies of pemetrexed that excluded patients with brain metastases. Though the median survival of over 14 months, PFS of 7.8 months and PR rate of 25% in the second-line setting might be a reflection of inadvertent favorable patient selection on these two non-randomized phase II trials, particularly given the large proportion of non-smokers and Asian/Pacific Islanders on the Stanford trial, these data certainly encourage further prospective exploration of this combination.
The treatments for advanced NSCLC have undergone revolutionary advances in the past decade, and these advances also benefit the treatment of brain metastases. With the discovery of driver mutations such as epithelial growth factor receptor (EGFR) and the consistent finding of superior efficacy of targeted therapies over chemotherapies in these patient populations, targeted therapies are now standard first-line treatments for patients with corresponding driver mutations, and they can have good CNS activity in treating brain metastases (23-25). It is often possible to treat active brain metastases with targeted therapies without the need for brain radiation, provided the patients are carefully monitored to ensure response.
Immunotherapy checkpoint inhibitors represent a new arena of treatment options for patients with advanced NSCLC, and three such agents—nivolumab, pembrolizumab, and atezolizumab—have been Food and Drug Administration (FDA)-approved for NSCLC in first and second line settings (26-32). Though none of these trials allowed patients with untreated brain metastases, CNS activity from checkpoint inhibitors has also since been reported (33,34).
Conclusions
With better understanding of CNS activities from systemic agents—including chemotherapies, targeted therapies, and immunotherapies—it is conceivable that the standard approach to treatment of stable brain metastases will shift toward replying on systemic therapies and less on radiation therapy (XRT). Future studies need not exclude, and should pay more attention to, this large segment of advanced NSCLC patient population with brain metastases.
Acknowledgements
Funding: The authors were supported by Genentech, Lilly, and Stanford NIH/NCRR CTSA (award number UL1 RR025744), and grants from Breathe California and the Colombo Trust.
Footnote
Conflicts of Interest: MA Gubens reports a consultancy relationship with Genentech not related to the current work; W Akerley reports past grant funding and fees for participation in review activities paid to his institution and past consulting fee or honorarium and travel support paid to him related to the current work, and past grants from the sponsors to his institution not related to the current work; CJ Langer reports an ongoing consultancy relationship with Lilly and Genentech not related to the current work; AD Colevas reports current grants or grants pending with Genentech to his institution not related to the current work; K Dragnev reports a past consultancy relationship with Genentech and Lilly not related to the current work; MA Socinski reports a grant to his institution to support the current work; HA Wakelee reports a grant to her institution to support the current work, as well as ongoing grants or grants pending to her institution not related to the current work. The other authors have no conflicts of interest to declare.
Ethical Statement: The studies (this is a pooled analysis of two trials) was reviewed by the IRB of each participating institution and written informed consent was obtained from all patients.
References
- Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005;23:6207-19. [Crossref] [PubMed]
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795-803. [Crossref] [PubMed]
- Chang JE, Robins H, Mehta MP. Therapeutic advances in the treatment of brain metastases. Clin Adv Hematol Oncol 2007;5:54-64. [PubMed]
- Oh Y, Stewart DJ. Systemic therapy for lung cancer brain metastases: a rationale for clinical trials. Oncology (Williston Park) 2008;22:168-78; discussion 178, 183, 188 passim.
- Edelman MJ, Belani CP, Socinski MA, et al. Outcomes associated with brain metastases in a three-arm phase III trial of gemcitabine-containing regimens vs. paclitaxel plus carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 2010;5:110-6. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 2001;19:843-50. [Crossref] [PubMed]
- Srivastava G, Rana V, Wallace S, et al. Risk of intracranial hemorrhage and cerebrovascular accidents in non-small cell lung cancer brain metastasis Patients. J Thorac Oncol 2009;4:333-7. [Crossref] [PubMed]
- Socinski MA, Langer CJ, Huang JE, et al. Safety of Bevacizumab in Patients With Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2009;27:5255-61. [Crossref] [PubMed]
- Fuld AD, Dragnev KH, Rigas JR. Pemetrexed in advanced non-small-cell lung cancer. Expert Opin Pharmacother 2010;11:1387-402. [Crossref] [PubMed]
- De Braganca KC, Janjigian YY, Azzoli CG, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol 2010;100:443-7. [Crossref] [PubMed]
- Besse B, Le Moulec S, Mazières J, et al. Bevacizumab in Patients with Nonsquamous Non-Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clin Cancer Res 2015;21:1896-903. [Crossref] [PubMed]
- Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:45-68. [Crossref] [PubMed]
- Akerley W, Hainsworth J, Oh Y, et al. Safety of bevacizumab therapy in subjects with brain metastases due to non-small cell lung cancer (NSCLC). J Thorac Oncol 2007;2:S467. [Crossref]
- Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2013;31:3926-34. [Crossref] [PubMed]
- Heist RS, Fidias P, Huberman M, et al. A Phase II Study of Oxaliplatin, Pemetrexed, and Bevacizumab in Previously Treated Advanced Non-small Cell Lung Cancer. J Thorac Oncol 2008;3:1153-8. [Crossref] [PubMed]
- Yano S, Shinohara H, Herbst RS, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res 2000;60:4959-67. [PubMed]
- Machein MR, Kullmer J, Fiebich BL, et al. Vascular endothelial growth factor expression, vascular volume, and, capillary permeability in human brain tumors. Neurosurgery 1999;44:732-40; discussion 740-1. [Crossref] [PubMed]
- Novello S, Camps C, Grossi F, et al. Phase II Study of Sunitinib in Patients with Non-small Cell Lung Cancer and Irradiated Brain Metastases. J Thorac Oncol 2011;6:1260-6. [Crossref] [PubMed]
- Dai H, Chen Y, Elmquist WF. Distribution of the novel antifolate pemetrexed to the brain. J Pharmacol Exp Ther 2005;315:222-9. [Crossref] [PubMed]
- Omlin A, D'Addario G, Gillessen S, et al. Activity of pemetrexed against brain metastases in a patient with adenocarcinoma of the lung. Lung Cancer 2009;65:383-4. [Crossref] [PubMed]
- Bearz A, Garassino I, Tiseo M, et al. Activity of Pemetrexed on brain metastases from Non-Small Cell Lung Cancer. Lung Cancer 2010;68:264-8. [Crossref] [PubMed]
- Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res 2012;18:938-44. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol 2015;10:232-6. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab vs. Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab vs. docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab vs. Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab vs. Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab vs. docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab vs. docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer 2016;98:114-7. [Crossref] [PubMed]


