Does VALIDATE-SWEDEHEART invalidate the use of bivalirudin in myocardial infarction?
Over the past two decades, technological advances in percutaneous coronary intervention (PCI) [such as 2nd generation drug eluting stents (DES), use of transradial access] and antithrombotic therapy (more potent P2Y12 agents) have made PCI safer and more effective in treating patients with acute coronary syndrome (ACS). The use of direct and indirect thrombin inhibitors along with glycoprotein IIb/IIIa inhibitors (GPI) during PCI have contributed to improved angiographic and clinical outcomes, but at the cost of increased bleeding complications and its attendant risks of morbidity and death (1).
Unfractionated heparin (UFH), an indirect thrombin inhibitor, had been the mainstay for procedural anticoagulation prior to the introduction of bivalirudin in the 1990s. Pharmaceutical-grade UFH is derived from mucosal tissues of porcine intestines or bovine lungs and works by indirectly binding to antithrombin III that inactivates free thrombin. The disadvantages of UFH include its inability to act on clot bound thrombus, potential for activation of platelets, and binding to tissue and plasma proteins thereby making its bioavailability, clearance, and dosing variable from patient to patient. Moreover, it has a nonlinear anticoagulant response at therapeutic range and may cause heparin-induced thrombocytopenia (HIT). UFH was commonly used in combination with a GPI in patients with ACS to further reduce the risk of myocardial reinfarction, stent thrombosis, and mortality, though this practice has been shown to increase hemorrhagic risks (2-4).
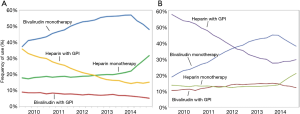
Bivalirudin is a direct thrombin inhibitor that has shown promise as an alternative to UFH. Bivalirudin is a synthetic peptide that overcomes many of the limitations seen with indirect thrombin inhibitors. It is a reversible inhibitor of thrombin and inhibits both circulating and clot-bound thrombin, in addition to inhibiting platelet activation and aggregation. Moreover, it does not bind to plasma proteins, has a predictable antithrombotic response, and does not carry the risk of HIT. Following two large randomized controlled trials, HORIZONS-AMI and ACUITY, which showed a reduced rate of adverse clinical events (primarily driven by lower major bleeding events) with bivalirudin compared with UFH with GPI, bivalirudin rapidly gained favor in the United States, surpassing the use of UFH as the anticoagulant of choice during PCI (5). These studies also led to the current NSTEMI and STEMI guidelines recommending bivalirudin as the preferred procedural anticoagulant for patients who are deemed to have higher risks of bleeding (Table 1) (6-9). In the United States, bivalirudin became the most frequently administered anticoagulant during PCI for NSTEMI and STEMI in 2009 and 2012 respectively (Figure 1).

Full table
However, the bleeding advantage seen with bivalirudin over heparin in ACS has been vigorously debated even prior to the most recent trial. First, the trials that showed a significant benefit for bivalirudin compared bivalirudin monotherapy to UFH with routine co-administration of a GPI, leading some to believe that the advantages associated with bivalirudin were largely driven by excess bleeding in the UFH arm due to concomitant GPI use. As guidelines and practices evolved to limit the use of GPI to “bail-out” or provisional use, more recent trials that compared bivalirudin to UFH monotherapy with minimal use of GPI subsequently showed an attenuation of the benefit of bivalirudin seen in prior trials. Second, the older trials used higher doses of UFH than is currently used in contemporary practice, leading to speculation that the advantages of bivalirudin were also driven in part by excess bleeding due to the higher doses of UFH used in those studies. However, in an analysis from the Evaluation of Drug-Eluting Stents and Ischemic Events (EVENT) registry, we showed that bivalirudin use during PCI was associated with a lower risk of bleeding at all comparator UFH doses (as measured by activated clotting time, ACT) without an increase in ischemic outcomes (10). Third, the largest controlled studies in favor of bivalirudin were conducted largely before the prevalent use of ticagrelor and prasugrel, higher potency P2Y12 inhibitors that lower the risk of ischemic complications after PCI (11,12). In two recent randomized trials that showed no difference in clinical adverse outcomes between bivalirudin and UFH with or without GPI, high potency P2Y12 inhibitors were administered prior to PCI in a significant proportion of patients (cumulatively 37% in MATRIX and 88% in HEAT-PPCI) compared to only clopidogrel in the HORIZONS-AMI and ACUITY (13,14). Finally, the prior trials that have shown a significant bleeding advantage of bivalirudin were in the era where PCI was predominately done via the femoral approach with minimal transradial access use. Radial artery access is associated with a lower risk of access site bleeding than with femoral access (15). In a single center study involving patients with STEMI where 85% of PCI was performed via transradial access, ticagrelor or prasugrel was the primary antiplatelet in 88% of patients and GPI was used in less than 15% of cases, HEAT-PPCI investigators found no difference in major bleeding though there was an increase in ischemic endpoints with bivalirudin when compared with UFH (14). Indeed, many of the studies that showed a favorable bleeding profile for bivalirudin also found small numerical increases in ischemic events, acute stent thrombosis, and unplanned revascularization when compared to heparin plus GPI.
In this context, the open-label randomized controlled trial VALIDATE-SWEDEHEART (Bivalirudin versus Heparin in ST-Segment and Non-ST-Segment Elevation Myocardial Infarction in Patients on Modern Antiplatelet Therapy in the Swedish Web System for Enhancement and Development of Evidence-based Care in Heart Disease Evaluated according to Recommended Therapies Registry Trial) was designed to address these conflicting uncertainties (16). In this registry-based randomized trial, 6,006 patients undergoing PCI for STEMI or NSTEMI were randomized to receive procedural anticoagulation with either bivalirudin or UFH alone. All patients were treated with high potency P2Y12 inhibitor (94.9% ticagrelor, 2.1% prasugrel, 0.3% cangrelor) before PCI. The trial excluded patients who were given GPI or where there was a prior plan to use GPI, though allowed for emergent unplanned use of these agents as rescue therapy. Consequently, a GPI was utilized in only 2.6% of patients. Additionally, in contrast to previous studies, the vast majority of PCIs (90.3%) were approached via radial artery access in both the bivalirudin and UFH arms. This trial, which reflect changes in PCI practices and antithrombotic therapies in recent years, showed that there was no difference in primary composite endpoint of death, myocardial infarction, or major bleeding between bivalirudin and UFH at 30 days (HR =0.89; 95% CI, 0.74–1.07; P=0.21) and at 180 days (HR =0.96; 95% CI, 0.83–1.10; P=0.54). The results were consistent across all prespecified patient subgroups including type of myocardial infarction (NSTEMI or STEMI), except for a strong trend towards benefit for bivalirudin in women (HR =0.78; 95% CI, 0.6–1.00; P=0.05).
The overall risk-benefit equipoise between bivalirudin and UFH demonstrated in this study should not be surprising. The authors of VALIDATE-SWEDEHEART propose that the predominant use of radial artery access as well as the low use of GPI attenuated the advantage of reduced bleeding seen previously with bivalirudin. In two large comparative analyses of patients with NSTEMI and STEMI using the National Cardiovascular Data Registry CathPCI, we found that reductions in bleeding risks with bivalirudin were largest among those undergoing transfemoral PCI. This improved bleeding safety effect of bivalirudin was significantly mitigated for patients who received radial artery access (17,18). These studies of real-world patients also showed that the safer bleeding profile of bivalirudin was diminished, though not completely eliminated, when adjusted for differential GPI use with UFH. Furthermore, while VALIDATE-SWEDEHEART was not powered to assess individual efficacy endpoints, it showed that there were no differences in rates of stent thrombosis or myocardial infarction. As suggested by the authors, this result may be due to the use of high potency P2Y12 inhibitors and/or the use of 2nd generation DES that have a lower rate of stent thrombosis compared with the stents used in HORIZON-AMI and ACUITY trials, although this hypothesis will need to be validated in randomized design.
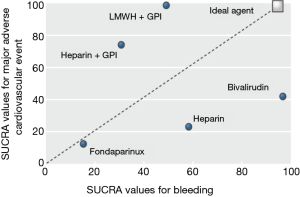
The VALIDATE-SWEDEHEART trial provides persuasive evidence that a strategy of radial artery access together with highly selective provisional use of GPI in PCI is preferred for patients at greater risks of bleeding and that this practice is not significantly associated with increased ischemic complications when high potency P2Y12 inhibitors are used. Nonetheless, while bivalirudin and UFH appear to be equivalent in terms of safety and efficacy in this trial, the question of which anticoagulant to use during primary PCI is far from settled. As shown in Figure 2, the ideal anticoagulant is one that will wholly balance the benefits of procedural anticoagulation in preventing ischemic events with the inherent risks of bleeding related to its use (5). While the results of VALIDATE-SWEDEHEART may alter the ranking plots of bivalirudin and UFH, the choice of anticoagulation will likely continue to be an individualized decision tailored to specific risk factors, antiplatelet agents, and PCI approaches available to each patient.
Finally, though bivalirudin has been available as a generic formulation in the United States since 2015, the acquisition costs of bivalirudin remain higher than for UFH. Prior analyses on the cost-effectiveness of bivalirudin compared to UFH were based on evidence suggesting lower rates of adverse events with bivalirudin (19,20). As advances in PCI technique and periprocedural management continue to alter the balance of ischemic to bleeding risks, additional randomized trials in addition to analyses of combined patient data and cost-effectiveness analyses are warranted to test individualized strategies for procedural anticoagulation in ACS. In the meantime, the use of UFH vs. bivalirudin should be individualized based on the risk of bleeding. Acquisition cost considerations may favor UFH, especially in patients undergoing PCI via the radial approach with provisional use of GPI. On the other hand, patients at high risk of bleeding, including those undergoing PCI via the femoral approach, planned GPI use, women and perhaps the elderly may benefit from bivalirudin use. The debate on UFH vs. bivalirudin use reminds us once again that one size fits all solutions do not lend themselves easily to patient care.
Acknowledgements
None.
Footnote
Conflicts of Interest: Sripal Bangalore, MD, MHA: Advisory board/consultant for The Medicines Company, Abbott Vascular, Pfizer, Astra Zeneca, Merck, and Amgen. C Ong has no conflicts of interest to declare.
References
- Verheugt FW, Steinhubl SR, Hamon M, et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv 2011;4:191-7. [Crossref] [PubMed]
- Bosch X, Marrugat J, Sanchis J. Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-ST segment elevation acute coronary syndromes. Cochrane Database Syst Rev 2013.CD002130. [PubMed]
- De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA 2005;293:1759-65. [Crossref] [PubMed]
- Kastrati A, Neumann FJ, Schulz S, et al. Abciximab and heparin versus bivalirudin for non-ST-elevation myocardial infarction. N Engl J Med 2011;365:1980-9. [Crossref] [PubMed]
- Bangalore S, Toklu B, Kotwal A, et al. Anticoagulant therapy during primary percutaneous coronary intervention for acute myocardial infarction: a meta-analysis of randomized trials in the era of stents and P2Y12 inhibitors. BMJ 2014;349:g6419. [Crossref] [PubMed]
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362-425. [Crossref] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:e344-426. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [PubMed]
- Bangalore S, Cohen DJ, Kleiman NS, et al. Bleeding risk comparing targeted low-dose heparin with bivalirudin in patients undergoing percutaneous coronary intervention: results from a propensity score-matched analysis of the Evaluation of Drug-Eluting Stents and Ischemic Events (EVENT) registry. Circ Cardiovasc Interv 2011;4:463-73. [Crossref] [PubMed]
- Stone GW, McLaurin BT, Cox DA, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006;355:2203-16. [Crossref] [PubMed]
- Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008;358:2218-30. [Crossref] [PubMed]
- Valgimigli M, Frigoli E, Leonardi S, et al. Bivalirudin or Unfractionated Heparin in Acute Coronary Syndromes. N Engl J Med 2015;373:997-1009. [Crossref] [PubMed]
- Shahzad A, Kemp I, Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 2014;384:1849-58. [Crossref] [PubMed]
- Subherwal S, Peterson ED, Dai D, et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention: a report from the National Cardiovascular Data CathPCI Registry. J Am Coll Cardiol 2012;59:1861-9. [Crossref] [PubMed]
- Erlinge D, Omerovic E, Fröbert O, et al. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. N Engl J Med 2017;377:1132-42. [Crossref] [PubMed]
- Secemsky EA, Kirtane A, Bangalore S, et al. Use and Effectiveness of Bivalirudin Versus Unfractionated Heparin for Percutaneous Coronary Intervention Among Patients With ST-Segment Elevation Myocardial Infarction in the United States. JACC Cardiovasc Interv 2016;9:2376-86. [Crossref] [PubMed]
- Secemsky EA, Kirtane A, Bangalore S, et al. Practice Patterns and In-Hospital Outcomes Associated With Bivalirudin Use Among Patients With Non-ST-Segment-Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention in the United States. Circ Cardiovasc Qual Outcomes 2017;10:e003741. [Crossref] [PubMed]
- Schwenkglenks M, Toward TJ, Plent S, et al. Cost-effectiveness of bivalirudin versus heparin plus glycoprotein IIb/IIIa inhibitor in the treatment of acute ST-segment elevation myocardial infarction. Heart 2012;98:544-51. [Crossref] [PubMed]
- Pinto DS, Stone GW, Shi C, et al. Economic evaluation of bivalirudin with or without glycoprotein IIb/IIIa inhibition versus heparin with routine glycoprotein IIb/IIIa inhibition for early invasive management of acute coronary syndromes. J Am Coll Cardiol 2008;52:1758-68. [Crossref] [PubMed]


