Enhanced recovery pathways in thoracic surgery from Italian VATS group: nursing care program
Introduction
The Italian video assisted thoracic surgery (VATS) group has developed a project called “Enhanced recovery after surgery (ERAS) and Fast Track in VATS Lobectomy” that incorporates the individual aspects of this work, with the aim of obtaining an ERAS protocol for thoracic surgery that is complete, easily to apply, and fit for today’s healthcare environment.
ERAS is an interprofessional, goal-directed program that begins in the preoperative period and extends through hospital discharge. The aim is to decrease perioperative stress, improve pain management and mobilization and minimize post-operative complications. This can lead to hastened patient recovery and reduced time in hospital. ERAS approach is multidisciplinary and requires the coordination of surgeons, nurses, anaesthesiologists, physiotherapists, dietitians (1). Programs typically include components such as patient assessment, exercise training, education, nutritional intervention, and psychosocial support.
This issue focalizes the role of nurses in ERAS program for patients submitted to Thoracic Surgery, in particular for cases of major lung resection. Although ERAS principles can be applied to open surgery too, they better fit to patients treated by a minimally invasive surgical approach (VATS).
The Italian VATS Group has a Registry, in which all VATS lobectomies carried out by accredited Italian centers are recorded; in addition to this and for the purpose of the aforementioned ERAS project, a dedicated and prospective ERAS Registry was created to validate specific ERAS indicators for minimally invasive thoracic surgery.
Herein ERAS nursing plan is described and a concrete work map is provided for nurses of Thoracic Surgery Units adhering to the project. The nursing staff is essential in each phase of the above mentioned project, as the nurse is the nearest figure to the patient. The closest contact with him is related to the time spent together, the number of meetings and telephone reports and the higher degree of confidence with him. The figure of the care provider is already, institutionally set; he/she is also called ‘Thoracic Surgical Nurse Specialist’ (TSNS), like at St James’s University Hospital, Leeds, UK (2).
The enhanced recovery pathway
Tables and checklists that the nursing staff should utilize for daily care and the indicators of the ERAS program implementation are included in the Supplementary.
Preadmission information, education and counseling
Patients should receive information in both written and oral form (digital supports like DVDs are useful, too). They should receive a diary that describes what they can expect to happen on each day after surgery. The diary has spaces for them to write down their progress and concerns.
Information provided by the nurse at the time of diagnosis are:
- Information on milestones of ERAS program.
- The principles of patient education: the nurse should ensure that patients and carers are aware of the importance of self-management in order to obtain a quicker recovery and to prevent postoperative complications.
- Information about the surgical procedure (fears about surgery are indications to call further meetings with the surgeon).
- The functioning and managing of chest drains.
- Information about anaesthesia and post-operative pain (emerging fears about this topic lead to request further interviews with anaesthesiologists). A detailed presentation is given with a particular focus on the reasons why pain control is required, how this is delivered and the potential side effects of medication. Sjöling et al. (3) reported that patient satisfaction with pain management is significantly correlated to the preoperative information received.
- Explanations on discharge criteria.
- Discharge advice, with regards to wound management, pain control, physiotherapy, driving and flying. Often questions are asked by patients and carers, about what type of care is required after surgery and if any additional help will be required at home.
Preadmission optimization
Patients should be prepared to the surgical treatment, both psychologically and physically, as for a sports competition. They should arrive at surgery while being at their physical optimum. Prehabilitation, defined as enhancement of the preoperative condition of a patient, has been proposed in order to augment functional (exercise) capacity before the surgical procedure, thus minimizing the postoperative morbidity and accelerating postsurgical recovery. The following items have to be tested, assessed and optimized by counseling or other actions, previous to surgery: smoking (if yes, counseling for cessation), alcohol (if yes, counseling for cessation), hyperglycemia (if yes, control blood glucose at a reasonable level), anemia (if yes, administer iron therapy, erythropoiesis-stimulating agents), mobility, dyspnea.
As for mobility, patient handling assessment is aimed to state if the patient is able to walk, stand up, go in or out of chair, toilet, transfer, move in bed etc. and if he/she does that independently or with the aid of auxiliary devices or carers. Handling assessment includes a personalized, mobilization program, too. For the latest topic (dyspnea), a patient unable to walk 3 flights at the stair test should be rigorously referred to physiotherapists for a more intensive rehabilitation program.
The physiotherapist (or often the nurse) should deliver, in this phase, an educational session on physiotherapy which includes advice on exercise prior to admission, the days following surgery and what to do when at home. Incentive spirometry (I.S.) devices should be given for preoperative exercise. The patients and carers are invited to take active part in the exercises with the physiotherapist/nurse, demonstrating the correct application of each exercise.
Preoperative preparation
- Avoid mechanical bowel preparation (excepted for patients with absence of defecation for more than 3 days).
- Prescribe fasten from solids 6 h prior to anaesthesia.
- Invite the patient to drink clear liquids (4,5) until 2 h before anaesthesia: it has been demonstrated to improve wellbeing.
Examples of clear liquids include, but are not limited to, water, fruit juice without pulp, carbonated beverages, carbohydrate–rich nutritional drinks, clear tea, black coffee (4).
As for carbohydrate drinks (not suited for diabetics), the amount expected is 800 ml the evening before and 400 mL 2 h before surgery. They are described to reduce nausea and vomiting and attenuate the increase in insulin resistance related to surgery (6).
- Administer LMW heparin 12 h before surgery.
- Avoid premedication (this is to reserve only to patients who refer at the interview a significantly high degree of anxiety before surgery).
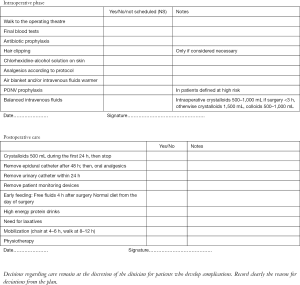
Intraoperative phase
It includes:
- Admission of the patient: the nurses invite the patient to walk to the operating theatre and they give to him/her all the support he/she needs; they make final blood tests, too.
- Administration of antibiotic prophylaxis prior to incision, if scheduled by the centre.
- Skin preparation: it includes firstly a shower (with plain soap), then hair removal only if necessary (by hair clipping in the operating room, immediately prior to surgery) and lastly the use of a skin antiseptic solution (preferably chlorhexidine-alcohol solution) (7).
- Thoracic epidural analgesia/multimodal analgesic strategies, performed according to the anesthetist’s guidelines
- Active warming (using air blanket and intravenous fluids warmer) in order to prevent intraoperative hypothermia. Hypothermia has been shown to impair drug metabolism, adversely affect coagulation, increase bleeding, cardiac morbidity and wound infection (8-10). Post-operative shivering also increases oxygen consumption and can worse pain (11).
- Post-operative nausea and vomiting (PONV) prophylaxis only in patients defined at high risk for PONV, according to a preoperative screening.
- Balanced intravenous fluids: intraoperative crystalloids 500–1,000 mL for surgery <3 h, otherwise crystalloids 1,500 mL, colloids 500–1,000 mL.
Postoperative care
- As for postoperative fluid management, administer crystalloids 500 mL during the first 24 h, then stop. With the commencement of oral diet and oral analgesia as soon as tolerated after surgery, post-operative intravenous fluids administration beyond 12–24 h is rarely needed. Indeed, intravenous fluids should be terminated within 24 h after surgery. In addition to a short duration of fluid therapy, enhanced recovery protocols reduce also the total volume of fluids (generally at about 500 mL). This is because a zero balance fluid regimen is associated with fewer cardiopulmonary complications (12). Moreover, balanced crystalloid solutions are preferred to 0.9% normal saline, in order to reduce flux across the extracellular space.
- Remove epidural catheter after 48 h; then, administer oral analgesics (paracetamol, for example).
- Remove urinary catheter within 24 h.
- Avoid or remove, as soon as possible, patient monitoring devices: arterial catheter, electrocardiographic electrodes, bracelet to measure blood pressure, patches on previous skin needle punctures, other monitoring wires, oxygen mask (replaced by nasal cannula if really necessary). This enhances patients’ early mobilization.
- Promote early feeding (defined as having oral intake of fluids or food within the first 24 h after surgery). It is generally recommended in all existing enhanced recovery programs. It begins with free fluids 4 h after surgery and hence, continue with normal diet from the day of surgery. Flavored high energy protein drinks are prescribed twice to three times a day. They are a useful ‘bridge’ to a normal diet, ensuring some protein and calorie intake early in the recovery process. However the existing evidences are weak, due to contrasting results, and further studies are needed.
- Promote early restart of the intestinal function. As for the prevention of ileus, laxatives are commonly used within enhanced recovery protocols, but no high quality data is available. Perioperative use of chewing gum (or alternatives, in edentulous patients) is shown to decrease ileus and length of stay (13).
- Promote mobilization within 24 h (in chair after about 4–6 h and walk at about 8–12 h, or in any case, as soon as tolerated).
For example:
- The patient will be helped to wear their garments soon, coming in the ward from the operating room.
- He will be early mobilized or placed sitting in a chair beside the hospital bed; the amplitude and frequency of the peripheral arterial pulse and the presence of perspiration will be detected. It may happen that during the first mobilization, the patient experiences fainting. In that case, the nurse will reassure and help him/her to go back to bed, and will try again later.
- At meal time, the patient will be invited to sit at the table.
- During the first mobilization, he will be invited to walk, short distances to get started, and afterwards greater distances, backed by health professionals or by a relative or by a walker (provided with oxygen, if necessary).
- By the first postoperative day (POD1), the ‘out of bed’ strategy should increase 6 h of duration per day, alternating sitting on chair with walking around. The patients will find on the diary the goals of mobilizations for each day and will write in what they have achieved. For its part, the nursing staff will ensure that the patients have the correct level of pain relief to make mobilization as comfortable as possible.
Ambulation and frequent position changes (first in bed and then out of bed) are central part of postoperative recovery programs, as they optimize ventilation and clear airway secretions; patient’s mobilization is considered an interdisciplinary teamwork responsibility. There is evidence to suggest that increasing physical activity prior to surgery contributes to improve patient outcomes (14,15). - Respiratory physiotherapy can improve postoperative dyspnea and health-related quality of life, with important psychosocial benefits. It comprises techniques that promote increasing lung volumes, as deep breathing exercises with or without devices (I.S.); other techniques focus on airway clearance, as coughing, postural drainage, percussion, vibration and shaking, if necessary. Furthermore, exercises for upper extremities and soft tissue release techniques are also used. The specific effects of respiratory physiotherapy after lung resection are the main topic of a recent protocol for systematic review (16) that will show the real importance of physiotherapy after lung resection. Probably, for Thoracic Surgery, this ERAS topic represents the most obvious difference from surgery of other body districts. It remains to be determined exactly which types of physiotherapy interventions are most effective. For example, as for I.S. in abdominal surgery, 2011 AARC guidelines (17) stated that “I.S. alone is not recommended for routine use in the preoperative and postoperative setting to prevent postoperative pulmonary complications…It is suggested that deep breathing exercises provide the same benefit as I.S.… Routine use of I.S. to prevent atelectasis in patients after upper-abdominal surgery is not recommended…”.
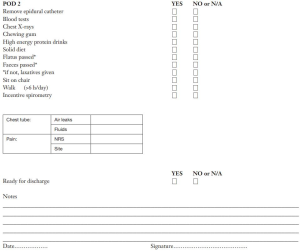
- In ERAS program the nurse has the power to decide the discharge of a patient with non-complicated outcome, on the basis of discharge criteria established by the surgeon in that center. These criteria may slightly vary according to center habits. Decision making for discharge is regulated by a protocol that takes into account air and fluid leaks in chest drains as well as clinical-radiological outcome. The above mentioned protocol chiefly depends on the type of pleural drainage used, water seal or digital, the latter being more objective, non-operator-dependent and therefore more prone to ERAS program. Generally the patient is discharged after removing the pleural drainage. In the event of prolonged air leaks, the surgeon will evaluate the possibility of leaving the patient out of the ERAS path or discharging him with the Heimlich valve. A provocative clamping could be useful, too. Obviously, at the moment of discharge, patients must have already received, by the nursing staff, whatever is necessary to complete their recovery at home.
- Follow-up is an important topic of ERAS nursing pathway, as it replaces the care given during conventional postoperative hospitalization. It has its own foundation in assistance by home carers or relatives, which should be identified in the preoperative phase, during preadmission counseling. The ERAS nurse should give the patients clear instructions about who to contact after discharge in case of any problem (possibly a 24-hour telephone helpline; if it is not practicable, there should be a local network involving also the general practitioner or other emergency services).
- For the first week, telephone follow up will be carried out by the nurse once daily: the patient will be interviewed about pain, dyspnea, pleural drainage if present, and will ask general questions about recovery (“I feel like this – is that right?”). If deemed necessary, the nurse will consult the surgeon to resolve some problems.
- Finally, a systematic audit, for example a bi-monthly meeting, is desirable, as staff need to evaluate the impact of what they do and should be encouraged to figure out how to best make ERAS fit their organization.
Conclusions
ERAS involves specific interventions at pre-operative, perioperative and post-operative point of care.
Nursing staff play a key role in the implementation of enhanced recovery protocols and a successful execution of the new pathway is related to a strict collaboration with the other healthcare professionals.
ERAS program includes radical changes in the structured working day of nursing staff, but also gives a new approach to evidence based care. The new way to care aims to optimize outcomes and improve patient experiences.
This is the reason why nursing staff must believe in the importance of ERAS pathway; their ability to adapt the program and to suit the variable local contexts enables their success.
Nursing workload, as Hübner et al. demonstrated in their study (18), is decreased by systematic implementation of an enhanced recovery protocol and the increasing compliance with ERAS protocol significantly correlates to decreasing nursing workload.
ERAS development create a culture in which teams can function well, team members flourish and patients receives the best care.
This protocol is based on the best available evidence in literature. Recommendations were made on the basis of existing guidelines (7,18-22), borrowed from other surgical disciplines. The practice of this protocol will help to realize if it needs to be modified on the basis of cardiopulmonary implications and peculiarities of thoracic surgery.
Italian VATS group
Enhanced recovery programme - Nursing care map
Acknowledgements
We would like to sincerely thank the Scientific Committee and the members of the Italian VATS Group, in particular Dr. Andrea Droghetti and Prof. Roberto Crisci for the scientific support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gotlib Conn L, McKenzie M, Pearsall EA, et al. Successful implementation of an enhanced recovery after surgery programme for elective colorectal surgery: a process evaluation of champions’ experiences. Implement Sci 2015;10:99. [Crossref] [PubMed]
- White J, Dixon S. Nurse led Patient Education Programme for patients undergoing a lung resection for primary lung cancer. J Thorac Dis 2015;7:S131-7. [PubMed]
- Sjöling M, Nordahl G, Olofsson N, et al. The impact of preoperative information on state anxiety, postoperative pain and satisfaction with pain management. Patient Educ Couns 2003;51:169-76. [Crossref] [PubMed]
- A American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anaesthesiologists Committee on Standards and Practice Parameters. Anaesthesiology 2011;114:495-511. [Crossref]
- Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2011;28:556-9. [Crossref] [PubMed]
- Smith MD, McCall J, Plank L, et al. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev 2014.CD009161. [PubMed]
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations — Part I. Gynecol Oncol 2016;140:313-22. [Crossref] [PubMed]
- Warttig S, Alderson P, Campbell G, et al. Interventions for treating inadvertent postoperative hypothermia. Cochrane Database Syst Rev 2014.CD009892. [PubMed]
- Scott EM, Buckland R. A systematic review of intraoperative warming to prevent postoperative complications. AORN J 2006;83:1090-104, 1107-13. [Crossref] [PubMed]
- Rajagopalan S, Mascha E, Na J, et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008;108:71-7. [Crossref] [PubMed]
- Wong PF, Kumar S, Bohra A, et al. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg 2007;94:421-6. [Crossref] [PubMed]
- Brandstrup B, Tonnesen H, Beier-Hogersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641-8. [Crossref] [PubMed]
- Ertas IE, Gungordulk K, Ozdemir A, et al. Influence of chewing gum on postoperative bowel activity after complete staging surgery for gynecological malignancies: a randomized, controlled trial. Gynecol Oncol 2013;131:118-22. [Crossref] [PubMed]
- Kerr A, Wotton R, Bishay E, et al. Rehabilitation for operated lung cancer programme: 18 months outcomes. Abstracts of the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery. Vienna, Austria. October 5-9, 2013.
- Spruit MA, Singh SJ, Garvey C. etal. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. [Crossref] [PubMed]
- Andersen KS, Skoffer B, Oestergaard LG, et al. The effect of respiratory physiotherapy after lung resection: protocol for a systematic review. Int J Surg Protocols 2017;4:1-5. [Crossref]
- Restrepo RD, Wettstein R, Wittnebel L, et al. Incentive spirometry: 2011. Respir Care 2011;56:1600-4. [Crossref] [PubMed]
- Hübner M, Addor V, Slieker J, et al. The impact of an enhanced recovery pathway on nursing workload: a retrospective cohort study. Int J Surg 2015;24:45-50. [Crossref] [PubMed]
- ERAS Society/Specialties/Nursing and AHPs/Care Plans. Available online: http://erassociety.org/
- Carmichael JC, Keller DS, Baldini G, et al. Clinical Practice for Enhanced Recovery after Colon and Rectal Surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017;60:761-84. [Crossref] [PubMed]
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations--Part II. Gynecol Oncol 2016;140:323-32. [Crossref] [PubMed]
- Ding J, Sun B, Song P, et al. The application of enhanced recovery after surgery (ERAS)/fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Oncotarget 2017;8:75699-711. [PubMed]