Lung cancer in connective tissue disease-associated interstitial lung disease: clinical features and impact on outcomes
Introduction
Interstitial lung disease (ILD), particularly idiopathic pulmonary fibrosis (IPF), is associated with an increased risk of developing lung cancer (LC). The incidence of LC among patients with IPF is estimated to range from 4.4% to 48% (1-4). Risk factors for the development of LC in IPF patients include older age, male sex, smoking, and combined pulmonary fibrosis and emphysema (CPFE) (5). The most frequent histological type of LC is squamous cell carcinoma, and LC is often located in peripheral fibrotic areas (6,7). LC is correlated with shorter survival in patients with IPF (6,7).
Connective tissue disease (CTD) represents a group of immunologically-mediated inflammatory disorders that affect a variety of organs. CTD includes rheumatoid arthritis (RA), systemic sclerosis (SSc), polymyositis and dermatomyositis (PM/DM), Sjögren’s syndrome (SS), systemic lupus erythematosus (SLE), and mixed CTD. Patients with CTD are highly susceptible to respiratory disorders, particularly, ILD. Like IPF, CTD-associated ILD (CTD-ILD) might be associated with an increased risk of LC. The occurrence of LC is estimated to range from 6.4% to 8.8% in CTD-ILD patients, and risk factors for LC in these patients include heavy smoking and the presence of emphysema (8,9). However, these data are based on a small number of cases, so the relationship between CTD-ILD and LC has not been fully investigated.
Herein, we performed a relatively large-scale, retrospective study to evaluate the prevalence, risk factors and clinical characteristics of LC in CTD-ILD patients and its impact on patient outcome.
Methods
Patients
This study was approved by our Institutional Review Board (IRB protocol #2105). We reviewed patients with CTD-ILD presenting to the Department of Respiratory Medicine, Kanazawa University Hospital from January 2003 to December 2016. CTD includes RA, SSc, PM/DM, SS, and SLE. Because these diseases overlap with each other in some cases, we expressed them as “components” of CTD. All cases were diagnosed by a rheumatologist, dermatologist or nephrologist using the appropriate classification criteria (10-14). All cases presented with ILD on chest high-resolution computed tomography (HRCT) scan. Patient baseline data at presentation, including smoking history, occupational exposure, types of CTD, chest HRCT findings, and pulmonary function test results were evaluated. The pattern of ILD on chest HRCT scan was evaluated by two pulmonologists (S Watanabe and K Saeki) in a blindly fashion according to the official ATS/ERS statement (15). The presence of emphysema was observed as well-demarcated areas of decreased attenuation and marked by a thin or no wall, and/or multiple bullae with upper zone predominance. During follow-up, data regarding treatment of CTD and incidence of pathologically proven LC were also collected. In cases with LC, histopathological findings, staging, location of primary lesions, and LC treatment regimens were examined. LC was classified according to the World Health Organization tumor classification, and staging of LC was performed using the 7th edition of the TNM classification of malignant tumors (16).
Statistical analysis
All statistical analyses were performed using SPSS software version 20 (IBM). Categorical variables were represented as counts and percentages, and were compared using the chi-squared test. Continuous variables were reported as median values ± standard deviation and interquartile range, and compared using the Mann-Whitney U test. Univariate logistic regression analysis was used to compare CTD-ILD in patients with and without LC. Variables that showed a significant difference were subjected to a multivariate logistic regression. Kaplan-Meier analysis was used to evaluate survival. P values <0.05 were considered statistically significant.
Results
Patient characteristics
The medical records of 268 patients with CTD-ILD in our hospital from 2003 to 2016 were retrospectively reviewed. Two patients were excluded from the study because they had a lung nodule or mass on chest CT scan without a pathological diagnosis. The remaining 266 patients were included in the present analysis. The median age was 60 years, and the median observation period was 64.0 months. Underlying CTDs included SSc (n=87, 32.7%), PM/DM (n=55, 20.7%), RA (n=53, 19.9%), SS (n=12, 4.5%), and others or overlapping CTDs (n=51, 19.2%).
Risk factors for LC
Of 266 patients with CTD-ILD, 24 (9.0%) were identified as having LC. Median follow-up was 43.5 months for patients with LC and 66.0 months for patients without LC. Compared to patients without LC, patients with LC were more likely to be male, older, heavy smokers, higher number of cigarettes, have RA, have a usual interstitial pneumonia (UIP) pattern and presence of emphysema on HRCT scan, have a lower predicted diffusing capacity of the lung carbon monoxide (DLco)%, and not be receiving immunosuppressive therapy (Table 1). Immunosuppressive agents included prednisolone, methylprednisolone, cyclophosphamide, cyclosporine, tacrolimus, methotrexate, and biological drugs. Multivariate analysis indicated that the presence of emphysema [odds ratio (OR), 8.473; 95% confidence interval (CI), 2.241–32.033] and nonuse of immunosuppressive therapy (OR, 8.111; 95% CI, 2.457–26.775) were independent risk factors for LC (Table 2).

Full table

Full table
Clinical features of LC
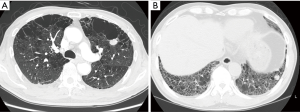
The clinical features of the 24 CTD-ILD patients with LC are shown in Table 3; details are provided in Table 4. In 20 patients, LC was diagnosed after the diagnosis of CTD-ILD (median duration, 9 months). The other 4 patients were diagnosed with LC at the time of CTD-ILD diagnosis, and 2 received LC diagnosis before CTD-ILD diagnosis. The primary site of LC was most frequently the peripheral lung area (91.7%). Tumors were located adjacent to fibrotic lesions in 14 (58.3%) cases, and emphysema or bullae were observed in 7 (29.2%) cases (Figure 1). The most common histological type was adenocarcinoma (n=10) followed by squamous cell carcinoma (n=9) and small cell LC (n=3). LC stages were as follows: stage I, 41.7%; stage II, 4.2%; stage III, 25.0%; and stage IV, 25.0%. Treatment of LC was based on staging and guidelines: 12 patients underwent surgery, 2 received chemoradiotherapy, and 10 received chemotherapy. Local recurrence after surgical resection developed in 2 (16.7%) of the 12 patients. Two patients with early-stage LC received best supportive care only because they had severe respiratory symptoms and a low performance status.

Full table
Full table
Outcome
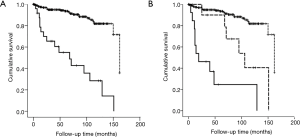
Of 266 patients, 41 (15.4%) patients died during the follow-up period. The most frequent cause of death was LC (n=13), followed by acute exacerbation (n=9), respiratory failure (n=4), infection (n=4), lymphoma (n=3), heart failure (n=3), and other (n=5). Among 24 patients with LC, 16 (66.7%) patients died and the median survival was 68.0 months. Of these 16 patients, 13 patients died from LC (81.3%), 2 from acute exacerbation (12.5%), and 1 from lymphoma (6.3%). One of the acute exacerbation cases occurred after chemotherapy. The survival rate was significantly lower among patients with LC than among those without LC (10-year survival rate: 28.5% vs. 81.8%; log-rank test, P<0.001) (Figure 2A). Based on the pathological stage, survival of patients with stage I/II LC or III/IV LC was significantly lower than that of patients without LC (P=0.001 and P<0.001, respectively). A significant difference was observed between the survival of patients with stage I/II LC and that of patients with stage III/IV LC (P=0.013) (Figure 2B).
Discussion
In the present study, we observed that LC was an important prognostic factor in patients with CTD-ILD. The clinical course of CTD-ILD is highly heterogeneous; some patients are stable for long periods, while others experience an accelerated clinical and functional decline (17). Life-threatening complications of ILD include acute exacerbation, respiratory failure, and secondary pulmonary arterial hypertension (18). However, the disease course and cause of death of CTD-ILD patients have not been fully examined. In our study, LC occurred in 9% of all study patients and was the most frequent cause of death among patients with CTD-ILD.
We identified two risk factors for LC in CTD-ILD patients. The first was the presence of emphysema, specifically, CPFE. Cottin et al. first characterized CPFE as a syndrome defined by the co-existence of emphysema in the upper lobes and fibrosis in the lower lobes on chest HRCT. All patients with CPFE were current or ex-smokers (19). Sugino et al. reported that the prevalence of LC among patients with CPFE is higher than that in patients with IPF alone (20). CPFE is also observed in patients with CTD-ILD and may be associated with an increased risk of LC (9). In the present study, most of the patients with LC with CPFE were current of ex-smokers: of 14 patients, 8 were current-smokers, 5 were ex-smokers, and 1 was a never-smoker. Although smoking is a major cause, CTD itself may also play a role in the development of emphysema (9,21). Another risk factor for LC was nonuse of steroids and immunosuppressants. Although there are no proven effective therapies for CTD-ILD, corticosteroids and/or immunosuppressive agents are commonly used as first-line therapy (22). The effects of immunosuppressive therapy on cancer risk remain controversial: some studies have suggested that immunosuppressants such as cyclophosphamide increase the risk of malignancy (23,24); however, other studies have not found this to be true (25,26). The present study is the first report to show that nonuse of immunosuppressant therapy may be associated with an increased risk of LC.
Although the pathogenic mechanisms underlying LC development in CTD-ILD remain unknown, chronic inflammation and a fibrotic environment are thought to contribute to tumorigenesis (27,28). The results of several studies have indicated that chronic inflammation can initiate tumor development by causing DNA damage or making cell susceptible to mutagens (29). Furthermore, inflammatory mediators, such as tumor necrosis factor, interleukin-1, interleukin-6, growth factors, chemokines, and proteases, can promote the development of cancer via proliferative, anti-apoptotic, and pro-angiogenic effects on the epithelium (30). Although just speculation, it is possible that a lack of immunosuppressive therapy might prolong inflammation, leading to repeated epithelial injury and promotion of the destruction, repair, and remodeling of lung tissue, resulting in the development of LC (31). The present results cannot demonstrate whether immunosuppressant therapy leads to improvement or stabilization of ILD resulting in the prevention of LC. Further prospective, long-term, observational studies are required to confirm this.
This study had some limitations. First, it was a retrospective single-institution study. Second, although the sample size was relatively large, the numbers of patients with RA and SS were smaller than those with SSc and PM/DM. This may have affected the prevalence of LC in CTD-ILD. Finally, patients presented to our hospital based on a diagnosis of LC. Thus, our study may have been affected by referral or selection bias.
In summary, LC occurs in 9% of patients with CTD-ILD. CPFE and nonuse of immunosuppressive therapy may be risk factors for LC. Uncontrolled inflammation as well as destruction of lung parenchyma, including fibrosis and emphysema, might contribute to an increased risk of LC. LC significantly impacts survival in patients with CTD-ILD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Kanazawa University Graduate School of Medicine Ethical Committee (ID: 2105) and we waved the requirement for patient consent because of the retrospective nature of this study.
References
- Turner-Warwick M, Lebowitz M, Burrows B, et al. Cryptogenic fibrosing alveolitis and lung cancer. Thorax 1980;35:496-9. [Crossref] [PubMed]
- Matsushita H, Tanaka S, Saiki Y, et al. Lung cancer associated with usual interstitial pneumonia. Pathol Int 1995;45:925-32. [Crossref] [PubMed]
- Wells C, Mannino DM. Pulmonary fibrosis and lung cancer in the United States: analysis of the multiple cause of death mortality data, 1979 through 1991. South Med J 1996;89:505-10. [Crossref] [PubMed]
- Ozawa Y, Suda T, Naito T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009;14:723-8. [Crossref] [PubMed]
- Usui K, Tanai C, Tanaka Y, et al. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology 2011;16:326-31. [Crossref] [PubMed]
- Lee T, Park JY, Lee HY, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: Clinical characteristics and impact on survival. Respir Med 2014;108:1549-55. [Crossref] [PubMed]
- Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015;147:157-64. [Crossref] [PubMed]
- Enomoto Y, Inui N, Yoshimura K, et al. Lung cancer development in patients with connective tissue disease-related interstitial lung disease: A retrospective observational study. Medicine (Baltimore) 2016;95:e5716. [Crossref] [PubMed]
- Cottin V, Nunes H, Mouthon L, et al. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum 2011;63:295-304. [Crossref] [PubMed]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. [Crossref] [PubMed]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581-90. [Crossref] [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. [Crossref] [PubMed]
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271-7. [Crossref] [PubMed]
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554-8. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Khanna D, Mittoo S, Aggarwal R, et al. Connective Tissue Disease-associated Interstitial Lung Diseases (CTD-ILD) - Report from OMERACT CTD-ILD Working Group. J Rheumatol 2015;42:2168-71. [Crossref] [PubMed]
- Antoniou KM, Margaritopoulos G, Economidou F, et al. Pivotal clinical dilemmas in collagen vascular diseases associated with interstitial lung involvement. Eur Respir J 2009;33:882-96. [Crossref] [PubMed]
- Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005;26:586-93. [Crossref] [PubMed]
- Sugino K, Ishida F, Kikuchi N, et al. Comparison of clinical characteristics and prognostic factors of combined pulmonary fibrosis and emphysema versus idiopathic pulmonary fibrosis alone. Respirology 2014;19:239-45. [Crossref] [PubMed]
- Cottin V, Freymond N, Cabane J, et al. Combined pulmonary fibrosis and emphysema syndrome in a patient age 28 years with severe systemic sclerosis. J Rheumatol 2011;38:2082-3. [Crossref] [PubMed]
- Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest 2013;143:814-24. [Crossref] [PubMed]
- Hill CL, Nguyen AM, Roder D, et al. Risk of cancer in patients with scleroderma: A population based cohort study. Ann Rheum Dis 2003;62:728-31. [Crossref] [PubMed]
- Abasolo L, Judez E, Descalzo MA, et al. Cancer in rheumatoid arthritis: occurrence, mortality, and associated factors in a South European population. Semin Arthritis Rheum 2008;37:388-97. [Crossref] [PubMed]
- Hesselstrand R, Scheja A, Akesson A. Mortality and causes of death in a Swedish series of systemic sclerosis patients. Ann Rheum Dis 1998;57:682-6. [Crossref] [PubMed]
- Bin J, Bernatsky S, Gordon C, et al. Lung cancer in systemic lupus erythematosus. Lung Cancer 2007;56:303-6. [Crossref] [PubMed]
- Hubbard R, Venn A, Lewis S, et al. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000;161:5-8. [Crossref] [PubMed]
- Bouros D, Hatzakis K, Labrakis H, et al. Association of malignancy with diseases causing interstitial pulmonary changes. Chest 2002;121:1278-89. [Crossref] [PubMed]
- Beyaert R, Beaugerie L, Van Assche G, et al. Cancer risk in immune-mediated inflammatory diseases (imid). Mol Cancer 2013;12:98. [Crossref] [PubMed]
- Rath T, Billmeier U, Waldner MJ, et al. From physiology to disease and targeted therapy: Interleukin-6 in inflammation and inflammation-associated carcinogenesis. Arch Toxicol 2015;89:541-54. [Crossref] [PubMed]
- Takiguchi Y, Sekine I, Iwasawa S, et al. Chronic obstructive pulmonary disease as a risk factor for lung cancer. World J Clin Oncol 2014;5:660-6. [Crossref] [PubMed]


