Understanding the possibility of image-guided thermal ablation for pulmonary malignancies
Introduction
There is a debate regarding the ideal treatment for inoperable patients with pulmonary malignancies. Although surgery has been the gold standard of care for centuries, other local therapies are applicable for patients with early stage non-small cell lung cancer (NSCLC) and oligometastatic disease from various types of malignancies. A variety of image-guided percutaneous thermal ablation technologies have been demonstrated as favorable therapeutic options for patients who are not surgical candidates because of poor cardiopulmonary reserve, anatomic constraints limiting resection, failure of traditional therapies, or refusal of operative approaches. Through case report series and small clinical trials, radio-frequency ablation (RFA), microwave ablation (MWA) and cryoablation have been demonstrated. These interventional thermal ablation techniques have been investigated, showing efficacy, safety, and good local disease control while preserving the normal lung parenchyma. However, we still have been without clinically relevant, statistically significant evidence for several years.
This is an analysis of the article by Mouli and colleagues, which was a review article of image-guided thermal ablation for patients with pulmonary malignancies (1). They evaluated various studies that have reported efficacy and safety for the treatment of both primary and metastatic disease.
Principle mechanism of thermal ablation
Before discussing the feasibility of current thermal ablation technologies, we recommend understanding the mechanism of action and local efficacy of each thermal ablation modality. Here, we summarized the comparison of image-guided percutaneous thermal ablation techniques in Table 1 (2). The unique characteristics of pulmonary parenchyma promote thermal ablation, including heat insulation and low electrical conductivity. These exclusive characteristics enable a larger volume of tissue to be ablated at a given energy level than in other organ tissues in the body (3). With RFA, an electrode from a generator causes frictional heating, elevating the tissue temperature to 60–100 °C. This heating effect creates a necrotic zone covering both the tumor and margin of normal lung parenchyma (4). As a weak point, this thermal energy can be limited by the heat-sink effect of adjacent blood vessels and airways (5). An important note for obtaining adequate margins is the ablation zone must exceed the tumor size (6). Therefore, RFA performs best for lesions smaller than 2 cm in diameter, lower success rates are seen with larger tumors (6-9). More specifically, a ratio of RFA-induced ground-glass opacity (GGO) to tumor area of greater than 4 (the bi-dimensional area on axial images) is correlated with a significantly higher rate of complete ablation than a ratio of less than or equal to 4 (6). Nowadays, new technologies have been developed to overcome the limitations of RFA by extending the volume of ablation or lowering convective cooling close to the bronchi or vessels. MWA uses microwaves to cause friction between water molecules, generating hyperthermia (10). Unlike RFA, during which only one probe is activated at a time, MWA enables simultaneous energy delivery with multiple probes, thus MWA can achieve larger ablation zones more quickly with less heat sink effect. Cryoablation uses compressed argon gas to generate subzero temperatures with the ice-ball formation effect. When temperatures are less than −40 °C, protein denaturation, cell rupture, and ischemia reactions occur (11). Unlike heat-based ablation, cryoablation does not create GGOs intra-procedurally rather; the ice ball is used to estimate the ablated margin. Most ablation protocols call for three freeze-thaw cycles to achieve tissue necrosis (12). Cryoablation involves inserting the probe into the tumor tissue providing moderate freezing and moving the probe away from accessible adjacent organs as necessary (13). An advantage of having the probe frozen to the tumor is that probe displacement will not occur during treatment as is the case with expandable RFA electrodes, which are very popular for lung ablation. Again, both MWA and cryoablation allow for simultaneous delivery of energy through several probes activated at the same time with a synergistic effect versus subsequent activation of the same probe. Large ablation volume gives improvement of local control of larger tumors and is under evaluation in clinical practice.

Full table
Clinical outcome of comparative study
The purpose of this author’s review was to demonstrate thermal ablation has shown safety and efficacy in the treatment of both NSCLC and oligometastatic disease in nonsurgical candidates (1). As the author mentioned in this article, one major limitation is that there have been no large randomized studies available to compare surgery with thermal ablation. Therefore, to ensure the availability of thermal ablation content, we investigated and summarized the clinical significance of thermal ablation for early stage NSCLC in Table 2 (14-20). Based on these studies, thermal ablation could be an alternative therapeutic modality to surgical treatment and stereotactic body radiation therapy (SBRT), specifically in selected patients with lung malignancies who have combined medical illnesses, who have limited lung function, and/or who are elderly. In the absence of randomized prospective trials, population based comparative studies may provide a higher level of evidence to guide clinical management decisions. Kwan et al. (17) examined survival in 1,897 patients with early stage NSCLC who underwent surgical resection or thermal ablation using the database from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. They sought to control for selection bias in their retrospective study using population-based data. After propensity score matching analysis was performed to reduce bias, overall survival and cancer specific survival were not significantly different between RFA and surgery. This study suggests that medical providers are appropriately selecting patients for thermal ablation based on factors such as age and presence of comorbid conditions.
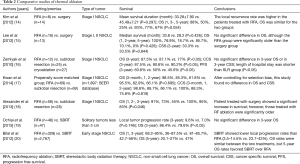
Full table
SBRT is an effective noninvasive interventional therapy with a good safety profile and remains the preferred treatment modality for patients who are unsuitable for surgery (21). Nevertheless, the disadvantage of SBRT includes the risk of radiation pneumonitis and fibrosis, especially in medically inoperable patients affiliated with interstitial lung disease or other reasons for borderline lung function. In addition, SBRT can cause bronchial stenosis if used to treat tumors in central lesions. SBRT is also limited by the fact that it generally requires five daily treatments, which is much more distressing to patients and their families than a single ablation procedure. Moreover, SBRT is expensive, with an average of $40,000 for the serial-treatment cost, including physician fees (18,21). When comparing the clinical outcomes of RFA with those of SBRT in patients with lung malignancies, RFA provided acceptable local tumor control and survival that were similar to using SBRT (19,20). By contrast, percutaneous thermal ablation therapies for peripheral lung cancers are associated with unacceptably high complication rates, primarily pneumothorax which make these techniques difficult to pursue as an alternative to SBRT. Another downfall of the percutaneous approach is that it is more painful and requires local anesthesia and/or sedation or general anesthesia, unlike SBRT. Therefore, the transthoracic thermal ablation techniques are relatively invasive and must compete with SBRT for effectiveness and safety to justify a place in the management algorithm for peripheral lung cancers. It should be clear and requires further investigation before being proven effective and safe for clinical applications.
Future direction of thermal ablation therapy
As previously discussed in this article, the most common technique for thermal ablation of pulmonary malignancies remains the percutaneous approach. In some patients with peripheral lung cancers and advanced underlying lung disease along with low pulmonary function, the risks of percutaneous RFA may be more acceptable than surgical resection. However, the risk of pneumothorax was not lower than SBRT as we mentioned before (1). Furthermore, percutaneous and transthoracic techniques have limitations in their approach for more centrally located tumors. The transbronchial approach can be more readily used to treat tumors located in the central portion of the lung, where the percutaneous approach may result in an unacceptably higher rate of pneumothorax and hemoptysis. If the incidence of complications could be reduced, more patients may benefit from thermal ablation therapy. The transbronchial approach for thermal ablation may potentially develop into a minimally invasive therapy with a low frequency of pneumothorax that can be used to treat patients with inoperable lung tumors located within either the central or peripheral areas of the lung. Hence, we have considered developing a new device for transbronchial thermal ablation. Coupled with advances in therapies, many of which can be delivered through the bronchoscope, we are entering a new era in which bronchoscopy may be used not only to diagnose lung malignancies but also to potentially treat lung malignancies. In fact, various transbronchial techniques have been used to treat central type endobronchial tumors. These techniques include heat modalities (argon plasma coagulation, electrocautery, laser phototherapy, photodynamic therapy), cold modalities (cryotherapy), or a direct injection of chemotherapeutic agents. In our laboratory, several new modalities are being investigated through ex vivo and in vivo animal experiments, with promising results (22,23). These thermal and non-thermal interventional therapies, and variations of these transbronchial techniques, are now being evaluated for the treatment of peripherally located lung tumors using the advanced diagnostic techniques already described. Given the illustrated antitumor effects of percutaneous thermal ablation of peripheral lung tumors and the hypothesis that transbronchial approaches have lower pneumothorax rates than transthoracic techniques, there have been concerted efforts to develop ablation technologies that can be delivered through the working channel of a flexible bronchoscope and endobronchial ultrasound (EBUS) system.
There have been only a few studies to develop bronchoscopy-guided RFA. Ten patients with stage IA lung cancer were treated using bronchoscopy-guided internally cooled RFA probes inserted within the tumors using CT imaging guidance in advance of planned surgical resection (24). The coagulation necrosis area increased with larger tips and longer ablation times, but the resected tissue contained residual tumor cells in all patients. Remarkably, except for two patients with mild chest pain, there were no complications such as bleeding or pneumothorax. In addition, they treated 23 peripheral lung lesions in 20 patients with early stage NSCLC using CT-guided bronchoscopic cooled RFA in their most recent paper (25). Local disease control was achieved successfully in most patients and there were no serious major complications reported.
A disadvantage of the transbronchial approach is that it can be difficult to reach and treat small, peripherally located tumors due to the lack of resources. As well as, transbronchial treatment may require intubation and anesthesia for patients. Given these recent efforts, it is credible that radial probe-EBUS using a guide sheath or electromagnetic navigational system may have a potential role in guiding thermal ablation for peripheral lung malignancies in patients who are not surgical candidates. The transbronchial techniques used to treat peripheral lung cancer may have significantly lower rates of complication, most notably a decreased incidence of pneumothorax, and would be favored over percutaneous interventional technologies. We believe that the optimal outline for successful bronchoscopic treatment of peripheral lung cancers would be to perform the diagnostic, staging, and therapeutic procedures in the same setting. This requires successful navigation to the targeted cancer with certainty of malignant diagnosis via rapid on site evaluation or frozen section to prevent the unnecessary treatment of non-malignant lesions it is also required for analysis of nodal sampling to exclude treatment of regionally advanced tumors. Emerging techniques such as intratumoral chemotherapy, target therapy, immunotherapy, and combinations of both with local ablative technologies, need to be investigated further in early-phase clinical trials to ensure safety and to confirm findings from preclinical studies of antitumor efficacy and synergy effects of combination therapy. Advanced therapies, such as local irradiation, heat and cold therapies, and gene based technologies, have brought the capability of potentially curing malignant disease without surgery when combined with the tools used in bronchoscopy to localize the tumor. These endoscopic techniques may provide fewer complications than transthoracic approaches when the same treatment modalities are applied.
Conclusions
In the past decade, there have been significant advances in technology that are facilitating the investigation of the therapeutic role of thermal ablation. In the future, it is likely that lung surgery for small-size oligometastatic lung disease will be replaced by minimally invasive techniques. The ideal therapy will have to demonstrate efficacy, tolerance, and cost effectiveness by prospective randomized control studies.
Acknowledgements
H Ujiie received a research scholarship from the Joseph M. West Family Memorial Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mouli SK, Kurilova I, Sofocleous CT, et al. The Role of Percutaneous Image-Guided Thermal Ablation for the Treatment of Pulmonary Malignancies. AJR Am J Roentgenol 2017;209:740-51. [Crossref] [PubMed]
- Lee KS, Pua BB. Alternative to surgery in early stage NSCLC-interventional radiologic approaches. Transl Lung Cancer Res 2013;2:340-53. [PubMed]
- Ihara H, Gobara H, Hiraki T, et al. Radiofrequency Ablation of Lung Tumors Using a Multitined Expandable Electrode: Impact of the Electrode Array Diameter on Local Tumor Progression. J Vasc Interv Radiol 2016;27:87-95. [Crossref] [PubMed]
- Alexander ES, Dupuy DE. Lung cancer ablation: technologies and techniques. Semin Intervent Radiol 2013;30:141-50. [Crossref] [PubMed]
- Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol 2001;12:1135-48. [Crossref] [PubMed]
- de Baère T, Palussière J, Aupérin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology 2006;240:587-96. [Crossref] [PubMed]
- Gillams AR, Lees WR. Radiofrequency ablation of lung metastases: factors influencing success. Eur Radiol 2008;18:672-7. [Crossref] [PubMed]
- Hiraki T, Sakurai J, Tsuda T, et al. Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a preliminary review of 342 tumors. Cancer 2006;107:2873-80. [Crossref] [PubMed]
- Okuma T, Matsuoka T, Yamamoto A, et al. Determinants of local progression after computed tomography-guided percutaneous radiofrequency ablation for unresectable lung tumors: 9-year experience in a single institution. Cardiovasc Intervent Radiol 2010;33:787-93. [Crossref] [PubMed]
- Robert Sheu Y, Hong K. Percutaneous lung tumor ablation. Tech Vasc Interv Radiol 2013;16:239-52. [Crossref] [PubMed]
- Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998;37:171-86. [Crossref] [PubMed]
- Hinshaw JL, Lee FT Jr, Laeseke PF, et al. Temperature isotherms during pulmonary cryoablation and their correlation with the zone of ablation. J Vasc Interv Radiol 2010;21:1424-8. [Crossref] [PubMed]
- de Baere T, Tselikas L, Woodrum D, et al. Evaluating Cryoablation of Metastatic Lung Tumors in Patients--Safety and Efficacy: The ECLIPSE Trial--Interim Analysis at 1 Year. J Thorac Oncol 2015;10:1468-74. [Crossref] [PubMed]
- Kim SR, Han HJ, Park SJ, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol 2012;81:395-9. [Crossref] [PubMed]
- Lee H, Jin GY, Han YM, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol 2012;35:343-50. [Crossref] [PubMed]
- Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg 2010;211:68-72. [Crossref] [PubMed]
- Kwan SW, Mortell KE, Talenfeld AD, et al. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non-small cell lung cancer. J Vasc Interv Radiol 2014;25:1-9.e1. [Crossref] [PubMed]
- Alexander ES, Machan JT, Ng T, et al. Cost and effectiveness of radiofrequency ablation versus limited surgical resection for stage I non-small-cell lung cancer in elderly patients: is less more? J Vasc Interv Radiol 2013;24:476-82. [Crossref] [PubMed]
- Ochiai S, Yamakado K, Kodama H, et al. Comparison of therapeutic results from radiofrequency ablation and stereotactic body radiotherapy in solitary lung tumors measuring 5 cm or smaller. Int J Clin Oncol 2015;20:499-507. [Crossref] [PubMed]
- Bilal H, Mahmood S, Rajashanker B, et al. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2012;15:258-65. [Crossref] [PubMed]
- Shah A, Hahn SM, Stetson RL, et al. Cost-effectiveness of stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. Cancer 2013;119:3123-32. [Crossref] [PubMed]
- Jin CS, Wada H, Anayama T, et al. An Integrated Nanotechnology-Enabled Transbronchial Image-Guided Intervention Strategy for Peripheral Lung Cancer. Cancer Res 2016;76:5870-80. [Crossref] [PubMed]
- Hirohashi K, Anayama T, Wada H, et al. Photothermal ablation of human lung cancer by low-power near-infrared laser and topical injection of indocyanine green. J Bronchology Interv Pulmonol 2015;22:99-106. [Crossref] [PubMed]
- Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest 2010;137:890-7. [Crossref] [PubMed]
- Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration 2015;90:47-55. [Crossref] [PubMed]


