Cardiorespiratory interaction with continuous positive airway pressure
Introduction
The cardiovascular and the respiratory systems closely interact, a change in ventilation impacts quickly on cardiovascular parameters. This symbiotic interaction is aimed at optimisation of ventilation by ensuring oxygen delivery to vital organs and removal of carbon dioxide. The cardiorespiratory system interacts at different levels, involving humoral, mechanical and neurological mechanisms.
Humoral interactions might include epinephrine from the adrenal glands and norepinephrine from the sympathetic nerves in response to hypoxia and hypercapnia, but it can also include nitric oxide, prostaglandins and other vasoregulatory peptides.
Mechanical interaction between the respiratory and the cardiovascular system are essential to maintain homeostasis. Pulmonary vascular resistance (PVR), for example, is heavily dependent on lung volume: when lung volume increases the alveolar expansion causes compression of the alveolar vessels and thus an increase of vascular resistance. Similarly, at low lung volumes, a more positive pleural pressure leads to a compression on the extra-alveolar vessels and, subsequently, to an increase in PVR (1). Negative inspiratory pressures during inspiration or high positive intrathoracic pressures in expiration might also influence the venous return.
Lastly, neurological mechanisms include various reflexes to coordinate respiratory muscle activity and autonomic responses of the brainstem (2). Peripheral interaction is mediated by the autonomous nervous system through the parasympathetic and sympathetic branches.
Under physiological conditions leading to homeostasis and eupnoea the cardiorespiratory interaction ensures an optimal transport of oxygen and maintains haemodynamic equilibrium. However, pathophysiological conditions may alter respiratory mechanics which can lead to impaired cardiovascular function. In conditions like obstructive lung disease, OSA or heart failure (HF), treatment with continuous positive airway pressure (CPAP) or non-invasive ventilation (NIV) might be required to maintain upper airway patency, control acute or chronic hypercapnic respiratory failure and ensure normal ventilation.
The effect of increased airway pressure on the cardiorespiratory system
Since its invention, CPAP has been used as effective treatment in cardiogenic pulmonary oedema, obstructive sleep apnoea (OSA) and in adult respiratory distress syndrome (ARDS). Positive airway pressure (PAP) causes an increased chest inflation, diminishes the development of atelectasis, recruits collapsed alveoli, decreases airway resistance, it reduces inspiratory effort and decreases of work of breathing (3).
Ventilatory effects
CPAP improves airflow by maintaining upper airway patency, it also helps chest inflation. PAP increases the pharyngeal cross-sectional area, whereas in the intrathoracic compartment it facilitates recruitment of collapsed alveoli (3). Previous studies on genioglossus electromyographic activity during CPAP use in asleep adults suggest that the acute effect of extrathoracic airway stenting is passive (4,5). Successive studies have indicate an additional and longer lasting effect of long-term CPAP therapy on the pharyngeal anatomy supporting the redistribution of extracellular water in the pharyngeal soft tissue and reducing soft tissue oedema (6-8).
CPAP increases functional residual capacity (FRC) (4,9) and reduces neural respiratory drive (NRD) (10), it shifts the functional volume on the pressure-volume curve to a more compliant part of the slope (11), resulting in a reduction of the work of breathing in patients with sleep-disordered breathing. CPAP also diminishes the work of breathing in congestive HF (11,12) and, moreover, the increased intrathoracic pressure forces fluid from the alveoli and the interstitial space back into the pulmonary circulation leading to an improved ventilation-perfusion ratio and better gas exchange (13).
Haemodynamic effects
Haemodynamic changes induced by PAP therapy are complex and data on accurate cardiorespiratory physiological studies are sparse. A direct measurement of many cardiovascular functions is cumbersome and, frequently, many physiological variables are measured using surrogate markers (e.g., transmural ventricular filling pressures).
Furthermore, the heart inside the chest represents a pressure chamber within a pressurised environment and accurate recordings of pressures in all associated compartments are difficult. However, the haemodynamic effects of positive-pressure ventilation can be described as processes that, by changing lung volume and intrathoracic pressure, affect cardiac preload, afterload or contractility.
Right and left ventricular function
The effects of positive pressure ventilation on the LV preload depend on changes in systemic venous return, RV output and LV filling.
Venous return is influenced by several factors such as vascular volume, venous compliance, resistance and the outflow pressure for the circuit, which is defined by the right atrial pressure (RAP). Venous return is maximal when the RAP equals zero, it is the main determinant of circulation equal to the left ventricular output under steady state conditions.
The right atrium is a highly compliant structure and the RAP resembles any variations in the intrathoracic pressure. An increase in the positive end-expiratory pressure (PEEP) by increasing lung volume decreases venous return by a diminished pressure gradient. This leads to decelerating venous blood flow, decreased RV filling and, consequently, diminished RV stroke volume (3).
The pump capacity of the right ventricle depends on RV filling volume (preload), RV contractility and the pressure, against which the right ventricle ejects, as well as the impedance and compliance of the arterial inflow bed (afterload).
An exact assessment of these parameters is difficult, because of uncertainties when calculating transmural pressures and the difficulties in obtaining adequate measurements of RV volumes due to its complex geometry. High pulmonary artery pressures increase the RV afterload limiting RV ejection (14).
A PEEP can modify PVR and the RV afterload by several mechanisms: firstly, it may impact on PVR by reducing an elevated pulmonary vasomotor tone caused by hypoxic pulmonary vasoconstriction. Recruitment of alveoli increases regional alveolar pO2 leading to diminished hypoxic pulmonary vasoconstriction, pulmonary vasomotor tone will fall and RV ejection will improve (15). Furthermore, a PEEP changes PVR by altering lung volumes. PVR is related to lung volume in a bimodal fashion, with resistance to flow being optimised near FRC. With increasing lung volumes from residual volume to FRC, PVR decreases and vascular capacitance increases.
In brief, the effects of PEEP on RV output depend on how PEEP changes lung volumes relative to normal FRC, the extent to which it can alleviate hypoxic pulmonary vasoconstriction, and the overall change in pulmonary arterial pressure (13).
A decrease in systemic venous return will result in reduced RV inflow. It will cause a decreased pulmonary venous return and inflow to the left ventricle as well, because the two ventricles pump in series. In addition, PEEP may have more direct mechanical effects on LV filling and, thus, on LV preload. PEEP-induced changes in lung volume and, in particular, regional lung volumes constrain the heart in the cardiac fossa.
In summary, LV preload during PEEP is predominantly affected by the decrease in systemic venous return and the decrease in RV output (series effects), while direct parallel interactions may have limited effects, unless in the presence of an acute cor pulmonale (16).
Left ventricular output
The pump capacity of the left ventricle depends on LV filling volume (preload), LV contractility and the pressure against which the left ventricle ejects (afterload). While PEEP decreases LV preload, its effect on LV contractility remains to be controversially discussed.
Positive pressure ventilation affects preload, afterload and ventricular compliance according to the Frank–Starling mechanism representing the relationship between stroke volume and end-diastolic volume. The stroke volume of the heart increases in response to an increase in the volume of blood in the ventricles prior to contraction (end-diastolic volume) when all other factors remain constant.
In contrast to its effect on the right ventricle, PEEP has been shown to decrease the LV afterload. It increases the pressure around structures in the thorax and, to a lesser extent, in the abdominal cavity, relative to atmospheric pressure. The remaining circulation is at atmospheric pressure and this effect results in a pressure differential, with most of the systemic circulation being exposed to lower pressure than the left ventricle and the thoracic aorta (17).
Thus, an increased intra-thoracic pressure, at constant arterial pressure, decreases the force necessary to eject blood from the left ventricle in a manner exactly analogous to decreased arterial pressure, at constant ITP (18).
Patients with HF are characterised by hypervolaemia, they are less sensitive to decreased preload. CPAP exerts its beneficial effect by reducing the elevation of sympathetic tone, thus affecting autonomic function in these patients. However, increasing cardiac surface pressure could lead to a decrease in coronary blood flow because of increased epicardial surface pressure and/or increased RAP. Tucker and Murray (19) reported decreases in myocardial blood flow out of proportion to decreases in myocardial work, suggesting that if PEEP led to a decreased coronary blood flow then it could jeopardise cardiac function when coronary flow reserve was limited, as in coronary artery disease; some caution should be paid when treating patients with active ischaemic heart disease with high levels of PEEP (16).
Acute effects of CPAP in awake patient
Although acute effects of CPAP in the awake patient have been extensively studied, most of the research available has focused on CPAP in pathological conditions rather than understanding its physiological effect in the healthy subject.
In fact, some of the first studies about CPAP involved infants with pulmonary oedema (20) and reports of adult patients with HF (21).
Obesity
Obesity has many effects on pulmonary mechanics, it increases intra-abdominal and intrathoracic pressures, reduces the transpulmonary pressure gradient leading to a hypo-inflated chest with low total lung capacity (TLC) and FRC (22), and leaving morbidly obese subjects breathing close to the residual volume. These effects lead to a high work of breathing and increased levels of NRD (10), awake and asleep.
In obese subjects, CPAP inflates the chest, increases FRC and counterbalances the intrinsic PEEP, particularly in supine posture (10), it reduces airway resistance and offloads the respiratory muscles (3), lowering NRD in addition to maintaining an open airway while asleep.
NRD, as measured by the electromyogram (EMG) of the diaphragm or the parasternal EMG (23), reflects the load on the respiratory system and is closely associated with breathlessness (24,25). In obese patients with OSA, CPAP titration effectively offloads the respiratory system in obese subjects, when awake, and reduces NRD by 30% during optimal chest inflation (26). However, when higher CPAP pressures are used, the chest hyperinflates, NRD increases again and patients become breathless. When BP is measured continuously with a beat-to-beat monitor, both BP and BPV rise acutely at increasing levels of CPAP pressure suggesting an up-regulation of the sympathetic nervous system (27).
NIV is used to treat patients with obesity hypoventilation syndrome (OHS); it improves gas exchange, quality of life and respiratory control. Studies in OHS have shown that NIV offloads respiratory muscles and reduces NRD (28). Held et al. retrospectively identified 18 patients with hypoventilation and pulmonary hypertension (PH) who were treated with NIV therapy. They assessed the pulmonary arterial pressure and cardiac function using right heart catheterization and echocardiography and found significant improvements in mean and systolic pulmonary artery pressure, PVR, right ventricular systolic function and improvements in walking distance at three-months follow up following treatment with NIV (29).
Acute decompensated HF
CPAP therapy is used in the treatment of decompensated HF or acute cardiogenic pulmonary oedema (ACPE) to improve lung volume recruitment, increase oxygenation, reduce work of breathing and increase cardiac output.
Effects on oxygenation
In patients with acute HF and pulmonary congestion lung compliance is impaired. Increased intrathoracic pressures help recruitment of collapsed alveoli, reverse atelectasis, and facilitate a fluid shift from the alveoli and the interstitial space into the pulmonary circulation; this decreases intrapulmonary shunting and improves gas exchange (30). CPAP and NIV achieve similar benefits with regards to oxygenation, work of breathing and cardiac output.
A recent trial showed that biPAP more rapidly improves oxygenation and dyspnoea scores, and reduces the need for ICU admission when compared to CPAP (31).
Effects on work of breathing
Work of breathing has only recently been recognised as a therapeutic target in patients with decompensated heart failure (DHF). DHF results in an increase in extravascular lung water, reduction in lung volume, and the total respiratory system compliance causes an increase in airway resistance. Work of breathing and oxygen requirements are increased in these patients which results in an imbalance between oxygen consumption and delivery.
In this context, PAP devices can decrease work of breathing and improve oxygenation; however, the effects on the haemodynamic system still remain largely undefined and, most importantly, it is still uncertain whether the beneficial effect of PAP results in improved cardiac function, improved oxygenation or in the relief of respiratory effort, or a combination of these.
Effects on cardiac output
Several studies have assessed the stroke volume in patients with DHF; a study of 9 patients with respiratory failure of cardiogenic origin found that although there was a significant decrease in the work of breathing while on CPAP, no relevant changes in stroke volume were noted (32). However, a reduction in the mean transmural filling pressures was observed, suggesting a better cardiac performance. When positive pressure was applied an increased pleural pressure limited the cardiac preload and the LV afterload explaining a drop in the systemic BP in such patients. However, pulmonary oedema can be accompanied by hypotension and shock making it difficult to use CPAP, and in such cases intubation may be required. However, continuous PAP delivered by a non-invasive interface reduced the need for intubation and mechanical invasive ventilation in patients with acute CHF; this conclusion was validated in a recent meta-analysis (33). The effects of bilevel positive airway pressure (BiPAP) are less well defined, a recent controlled comparison of BiPAP versus CPAP had to be terminated because of increased risks of myocardial infarction in the BiPAP group, despite more rapid improvements in ventilation and vital signs (34).
Despite the evidence showing a beneficial effect of both CPAP and BiPAP in patients with acute DHF current guidelines remains ambiguous; although all major guidelines suggest the use of PAP therapy the levels of evidence, when presented, vary from Class Ia, Level A to Class IIa, level B (Table 1).

Full table
Acute effects of CPAP in the asleep patient
OSA
OSA is a chronic condition characterised by repeated interruption of ventilation during sleep due to upper-airway collapse, leading to periodic apnoea and hypopneas, hypoxaemia, increased intrathoracic pressure swings, arousal from sleep and sleep fragmentation. OSA syndrome is diagnosed when the number of apnoeas and hypopneas (Apnoea-Hypopnoea Index, AHI) are at least 5 per hour of sleep (AHI >5) and the patient presents with symptoms of excessive daytime sleepiness (40,41).
Besides symptomatic presentation, OSA impacts on several extrapulmonary functions, like BP control, sympathetic nervous system activity (SNA), endothelial and vascular function (42-44). It is frequently associated with hypertension, this is in part because of the common underlying risk factors, but also because of a possible causative link between intermittent hypoxia, chemoreceptor and baroreflex stimulation, activation of sympathetic and renin-angiotensin system as possible pathophysiological mechanisms. Notably, chemoreflex and baroreflex dysfunction and sympathetic activation is present not only during sleep but also during wakefulness (45).
Whereas long-term use of CPAP might improve BP control, any effects are moderate and probably more notable in subjects with severe OSA and uncontrolled hypertension (46,47); available studies on acute effects of CPAP, although conducted on small populations, support a decrease in sympathetic activity (48,49), while some studies describe a protective effect on BP, in particular on the systolic BP (50,51). Beyond the effect on BP control, several studies suggest that the acute effect of CPAP may reduce BP variability while patients sleep (52). In a study evaluating the effects of a short-term (2 weeks) treatment with autotitrating CPAP systolic and diastolic BP variability, measured as standard deviation of three BP measurements, were reduced especially in hypertensive patients (53).
Vascular function
Several studies have been conducted in order to investigate the effect of OSA on endothelial function. However, because of the different methods and sites of evaluation of the endothelial function results are not easily comparable: one study evaluating forearm blood flow showed an altered endothelial function of large conduit artery in OSA patients after graded brachial artery infusion of acetylcholine and sodium nitroprusside; a different study was unable to detect an impairment in microvascular endothelial function, measuring the reactive hyperaemia index by tonometry, in diabetic patients with different grades of OSA (54).
However, a systematic review and meta-analysis by Schwarz and colleagues found that in 4 RCTs comparing the effects of therapeutic CPAP versus subtherapeutic CPAP (or no intervention) on endothelial function, CPAP therapy (range, 2–24 weeks) significantly increased absolute % flow-mediated dilatation by 3.87% (55). CPAP is thought to improve endothelial function in OSA. Few studies have investigated the acute effect of CPAP on endothelial function in subjects with OSA. A small study documented an improvement in endothelial function, assessed by the flow-mediated dilation (FMD) technique, following 1 week of CPAP in patients with severe OSA, but the effect rapidly vanished after CPAP withdrawal (56). Moreover, a prospective study on 30 subjects with OSA indicated an improvement in FMD after an overnight use of nasal CPAP (57). The improvement on endothelial function exerted by CPAP could be acute but reversible.
Nitric oxide (NO) is a cellular signaling molecule and the major determinant of endothelial function and vasodilation. However, little is known about NO in OSA patients, but some studies suggest a reduction in NO availability measured as serum nitrites and nitrates in subjects with moderate/severe OSA (58,59), which can be rapidly restored by applying CPAP (58,60).
Several studies have investigated the association of OSA with arterial stiffness; a systematic review tried to summarise the results coming from 24 observational studies or clinical trials which assessed arterial stiffness by measuring carotid-femoral pulse wave velocity (PWV), brachial-ankle PWV, the augmentation index (AI) or ultrasound. The authors of the meta-analysis concluded that OSA is likely to be an independent risk factor for an increase in vascular stiffness, assessed by daytime, and suggest a possible direct correlation between the severity of arterial stiffness and OSA (61).
The acute effects of apnoeic episodes on vascular distensibility have not entirely been established; a prospective cross-sectional study showed an acute increase in arterial stiffness, measured as arterial AI by applanation tonometry, during obstructive apnoeas recorded by nocturnal polysomnography (62).
Little is known about the acute effect of CPAP in this context during ongoing treatment. Some observations indicate a possible beneficial effect of CPAP on arterial stiffness. Indeed, an improvement in vascular stiffness evaluated by pulse wave analysis early in the morning after CPAP therapy has been described, when compared with the same measurements in the afternoon (63). Another study concluded that CPAP improves vascular function even after short-term treatment (64), but also that there is a rapid reversal following CPAP withdrawal (65).
HF
OSA is common in patients with HF, the prevalence can be up to 53% considering a diagnostic AHI of 10 events/hour (66), but the prevalence vary greatly according to different AHI cutoffs and definitions. Repeated apnoeas in these patients expose the cardiovascular system to a cascade of intermittent hypoxia, large negative intrathoracic pressure changes, surges in SNA and BP, and frequent arousal from sleep, all of which may have adverse cardiovascular consequences (67).
Interestingly, the relationship between HF and OSA is bivariate: although OSA probably contributes to the development or progression of HF, HF might also contribute to the development of OSA as fluid retention in the legs while upright during the day shifts into the neck when recumbent during sleep. Such fluid displacement might cause distension of the neck veins and/or edema of the peripharyngeal soft tissue that increases tissue pressure, predisposing to pharyngeal obstruction and OSA (68,69).
OSA alters the physiologic cardiovascular quiescence during sleep characterised by a decrease of the metabolic rate, sympathetic nervous activity, BP, and heart rate; Apnoeas and hypopneas cause intermittent surges in SNA, BP, and HR with potentially adverse consequences (45).
Furthermore, during obstructive apnoeas, negative inspiratory intrathoracic pressures generated against the upper airway occlusion increases venous return to the right heart causing, together with OSA induced hypoxic pulmonary vasoconstriction, right ventricular distension towards the left heart thus impeding LV filling (70,71). This combination of increased LV afterload and diminished LV preload reduces stroke volume and cardiac output more in patients with HF than in healthy subjects. Increased LV transmural pressure also increases myocardial oxygen demand while simultaneously reducing coronary blood flow while apnoea-related hypoxia limits oxygen delivery (71). These conditions can precipitate myocardial ischaemia and impair cardiac contractility and diastolic relaxation.
CPAP acutely alleviates OSA, abolishes negative intrathoracic pressure swings, and reduces nocturnal BP and HR, resulting in reduced LV afterload. This cardiac unloading is accompanied by improved myocardial oxidative metabolism (72).
Long-term effects of CPAP therapy
OSA
Observational studies, clinical trials and successive meta-analyses have shown a significant effect of CPAP therapy on BP control in patients with OSA. In 2014, a meta-analysis of 31 randomized controlled trials by Fava and colleagues showed a significant but moderate BP lowering effect of CPAP in patients with OSA, the extent of reduction was 2.6±0.6 for the systolic and 2.0±0.4 mmHg for the diastolic BP, with a slightly stronger effect at night. The analysis indicated that the severity of OSA was associated with a stronger impact of CPAP on systolic BP (47). The beneficial effect of CPAP on BP and the effect size were confirmed by successive meta-analyses (73,74). Patients with severe OSA or treatment-resistant hypertension were reported to have the most clinically relevant effects. Indeed, two meta-analyses of RCTs focuses on OSA patients and treatment-resistant hypertension, they reported a decrease of −4.78 and −2.95 mmHg in systolic and diastolic BP, and of −7.21 and −4.99 mmHg, respectively (75,76).
OSA might be associated with an increase in plasma aldosterone which plays a pivotal role in the metabolism of electrolytes and fluid balance, as well as in BP control. Plasma aldosterone levels have been associated with severity of OSA in hypertensive patients and, in particular, in subjects with resistant hypertension (77,78). Although a causal link between hyperaldosteronism and OSA has not been proven so far, it has been reported that mineralocorticoid receptor antagonists exert a protective effect on OSA patients by reducing the aldosterone-mediated chronic fluid retention. Some studies have investigated the effect of CPAP on aldosterone levels, the results could not find a reduction in aldosterone levels following CPAP treatment in patients with OSA. However, in a study of 117 patients with resistant hypertension and moderate-to-severe OSA, a long-term effect of CPAP on aldosterone secretion has been found. Moreover, patients with optimal CPAP usage (an average of more than 4 hours per night) for 6 months presented with a lower aldosterone excretion, especially if baseline BP was elevated (79). To the contrary, this has not been confirmed in other interventional or observational studies (80). A recent meta-analysis on a small overall sample size could not support the beneficial effect of CPAP therapy on plasma aldosterone levels (81).
Several studies were conducted to assess the effect of long-term CPAP therapy on endothelial function in patients with OSA, as assessed by FMD of the brachial artery. The results, although limited by a small sample size and a relative short duration of the treatment (<6 months), support a protective effect of CPAP on endothelial function (82,83). A small prospective interventional study confirmed the positive effect but showed a rapid reversal (64).
Few studies indicated a possible increase in NO availability by long-term use of CPAP in patients with moderate to severe OSA (84). Interestingly, these studies also detected a reduction in apoptotic endothelial cells, which are an ex vivo marker of vascular damage, following CPAP therapy; hypothetically, this could indicate a link between OSA and vascular dysfunction through endothelial cell apoptosis (85). A recent meta-analysis showed a significant improvement of endothelial function, as assessed by FMD, in subjects with OSA after at least one month of CPAP treatment (86). In order to better understand the independent impact of CPAP on endothelial function, studies including larger populations and long-term follow up of CPAP use as well as trials investigating the effect of other conventional cardiovascular risk factors, beside OSA, on vascular function would be useful.
In recent years, several studies have assessed the effects of long-term CPAP on arterial function using different methods, like PWV, cardio-ankle vascular index (CAVI), AI or aortic elastic parameters by echocardiography, thus limiting the possibility to directly compare the results. Using PWV, interventional and observational studies report a beneficial effect of chronic CPAP therapy (87,88), a result which was confirmed by a meta-analysis (89). Moreover, preliminary data indicate a possible protective effect of long-term use of CPAP in patients with OSA on several other indices of vascular stiffness, like the beta coefficient obtained by CAVI (90), AI and augmentation pressure (AP) obtained by radial tonometry (91), as well as aortic strain and distensibility measured by echocardiography (92); again, the results were confirmed by meta-analysis despite a low number of included studies and the non-randomized design of studies (61). Interestingly, the protective effects of CPAP on arterial stiffness detected within 6 months of treatment has not been confirmed during longer periods of treatment (88), thus requiring further investigations in order to understand the extended long-term consequences of CPAP on vascular function.
Moreover, an association of carotid artery stiffness with the AHI has been reported in obese children (93), suggesting that vascular remodelling might begin even as early as in childhood.
HF
Chronic effects of PAP in patients with HF and left ventricular dysfunction have been studied mostly in conjunction with sleep-disordered breathing. Wang and colleagues (94) reported in a series of 164 prospectively enrolled patients with HF that mortality was higher in those with untreated sleep apnoea when compared with subjects with mild or no sleep-disordered breathing after controlling for confounding factors. Apneic episodes in patients with HF can be both obstructive and central in nature. Central sleep apnoea is caused by the complete or incomplete reduction of airflow, accompanied with a consensual change in neuro-respiratory drive. This is caused by nocturnal hyperventilation that characterises patients with HF (95).
As discussed above, HF leads to increased left ventricular filling pressure. The resulting pulmonary congestion activates lung vagal receptors that stimulate hyperventilation and hypocapnia. Superimposed arousals cause further abrupt increases in ventilation and drive PaCO2 below the threshold for ventilation, triggering a central apnea. Central sleep apneas are sustained by recurrent arousals resulting from apnea-induced hypoxia and the increased effort to breathe during the ventilatory phase because of pulmonary congestion and reduced lung compliance. Although central apneas have a different pathophysiology than obstructive apneas and are not associated with the generation of exaggerated negative intrathoracic pressure, they both increase SNA (96).
Several studies have treated sleep apnoea with CPAP in the hope to reverse left heart remodelling: Kaneko and colleagues (97) showed that CPAP applied for one month improved LVEF by 9% and reduced systolic BP in patients with OSA and HF. Mansfield et al. (98) replicated the same findings but with inconsistent changes on BP. However, few data are available on mortality. A trial by Bradley and colleagues randomised 258 patients with HF and sleep-disordered breathing to optimal care plus CPAP versus optimal care alone. Although there was no survival benefit seen over 24 months, the AHI and overnight SpO2, LVEF, 6-min walk distance and plasma norepinephrine all improved with CPAP (99).
While the focus of attention was CPAP in the 1990s, adaptive servo-ventilation (ASV), which changes the airway pressure according to the breathing pattern, has been investigated more recently. Following a series of small trials with ASV in HF, a meta-analysis compared ASV with best care and reported ASV to be associated with improved AHI, LVEF, 6-min walk distance and survival (100).
Recently, the SERVE-HF trial (101) enrolled 1,325 patients with symptomatic HF with LVEF <45% and central sleep apnoea (CSA, AHI >15, >50% central) from 91 centres who were randomised to usual care and ASV or usual care only and followed for a mean of 31 months. The main outcome, a composite end point (death and cardiovascular events), was not different between groups. However, ASV was associated with an unexpected increase in all-cause and cardiovascular death, which appeared to occur out of hospital (possibly as a result of sudden cardiac death) and was more pronounced in the patients with lower LVEF.
Compliance with ASV in the SERVE-HF trial was limited (3.4 h per night) but reflected clinical experience, and 29% of patients randomised to ASV dropped out. About 16% of the control patients swapped to ASV, thus making the ‘intention to treat’ analysis difficult.
Another study, the ADVENT-HF trial, is still recruiting; it focuses on the effects of ASV with different algorithms and pressure settings in patients with HF who have a mixture of OSA and CSR-CSA (NCT01128816). The primary composite outcome is death or first cardiovascular hospital admission or new onset atrial fibrillation/flutter requiring anti-coagulation but not hospitalisation or delivery of an appropriate shock from an ICD not resulting in hospitalisation. The main difference between the ADVENT-HF trial and other trials in the field, for example the SERVE-HF trial, are methodological in nature. In fact, ASV devices use different algorithms to suppress CSA and it is difficult to determine whether the results of the SERVE-HF trial were a class effect of ASV or specific to the ASV device used.
Furthermore, compliance to PAP devices remains a problem: in the SERVE-HF trial 29% of patients either discontinued or never used ASV, while 16% of patients randomly assigned to control crossed-over to positive airway pressure therapy. ASV compliance was low, averaging only 3.4 h per night 1 year after randomisation. This low adherence suggests that subjects remain exposed to CSA during a substantial period when ASV was not worn. In addition, a potential reason for low compliance was that 76% of treated subjects used a full face mask, which is generally less well tolerated than a nasal interface. The ADVENT-HF trial will help to answer some of these questions, enrolling also non-sleepy patients with OSA and performing a close follow up (6 months vs. 1 year follow up).
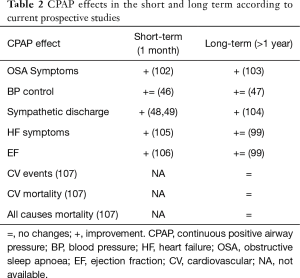
In conclusion, CPAP benefits in the short and long term vary consistently according to different CPAP effects (Table 2). A recent systematic review and meta-analysis (108) showed that the use of PAP, compared with no treatment or sham-CPAP, was not associated with reduced risks of cardiovascular outcomes or death for patients with sleep apnoea. However, a meta-analysis by Abuzaid and colleagues confirmed similar findings, underlining the role of treatment compliance with a daily CPAP usage greater than 4 hours per night (109). Considering the findings of the SAVE trial (107), current evidence confirms that CPAP for OSA improves symptoms and related conditions (e.g., OSA related hypertension) but it does not necessarily prevent cardiovascular events.
Full table
Conclusion and future perspectives
The cardiovascular and the respiratory systems interact closely. Positive airway pressure, as provided by CPAP therapy, is a non-pharmacological treatment that, in the acute setting, not only supports the respiratory system but also improves cardiac output in patients with HF; the current evidence supports the use of CPAP in the awake patient with decompensated HF. CPAP therapy is also associated with a reduction in hospital mortality, intubation rates and ICU length of stay.
In the asleep patient, CPAP can prevent airway obstructions and ensure an adequate oxygenation. Furthermore, CPAP normalises blood pressure surges caused by the sympathetic nervous system in patients with OSA. This ensures a normalisation of the nocturnal blood pressure profile with a physiological dipping pattern.
In the long term, CPAP use in sleep-disordered breathing has been shown to improve cardiovascular parameters like blood pressure control and heart rate but its impact on mortality and cardiovascular events is still debated.
Further randomised controlled clinical trials are necessary to clarify long-term outcomes and to define suitable parameters to understand variables that impact on cardiovascular risk in OSA patients. In parallel, observational cohort studies might be useful to better understand the physiological phenomena in patients treated with CPAP, as beneficial or unexplained findings from large clinical trials warrant a deeper understanding of the underlying pathophysiological mechanisms.
Acknowledgements
Dr. Steier’s contribution was partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rhoades RA, Bell DR. Medical Phisiology: Principles for Clinical Medicine [Medical Physiology (Rhoades)]. Philadelphia: Lippincott Williams & Wilkins; 2012;840.
- Scharf SM, Pinsky MR, Magder S. editors. Respiratory-Circulatory Interactions in Health and Disease. New York: Marcel Dekker, 2001.
- Kato T, Suda S, Kasai T. Positive airway pressure therapy for heart failure. World J Cardiol 2014;6:1175-91. [Crossref] [PubMed]
- Alex CG, Aronson RM, Onal E, et al. Effects of continuous positive airway pressure on upper airway and respiratory muscle activity. J Appl Physiol (1985) 1987;62:2026-30. [Crossref] [PubMed]
- Deegan PC, Nolan P, Carey M, et al. Effects of positive airway pressure on upper airway dilator muscle activity and ventilatory timing. J Appl Physiol (1985) 1996;81:470-9. [Crossref] [PubMed]
- Ryan CF, Lowe AA, Li D, et al. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis 1991;144:939-44. [Crossref] [PubMed]
- Schwab RJ, Pack AI, Gupta KB, et al. Upper airway and soft tissue structural changes induced by CPAP in normal subjects. Am J Respir Crit Care Med 1996;154:1106-16. [Crossref] [PubMed]
- Corda L, Redolfi S, Montemurro LT, et al. Short- and long-term effects of CPAP on upper airway anatomy and collapsibility in OSAH. Sleep Breath 2009;13:187-93. [Crossref] [PubMed]
- Verbraecken J, Willemen M, De Cock W, et al. Continuous positive airway pressure and lung inflation in sleep apnea patients. Respiration 2001;68:357-64. [Crossref] [PubMed]
- Steier J, Jolley CJ, Seymour J, et al. Neural respiratory drive in obesity. Thorax 2009;64:719-25. [Crossref] [PubMed]
- Naughton MT, Rahman MA, Hara K, et al. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation 1995;91:1725-31. [Crossref] [PubMed]
- Granton JT, Naughton MT, Benard DC, et al. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med 1996;153:277-82. [Crossref] [PubMed]
- Kaye DM, Mansfield D, Naughton MT. Continuous positive airway pressure decreases myocardial oxygen consumption in heart failure. Clin Sci (Lond) 2004;106:599-603. [Crossref] [PubMed]
- Tulevski II, Romkes H, Dodge-Khatami A, et al. Quantitative assessment of the pressure and volume overloaded right ventricle: imaging is a real challenge. Int J Cardiovasc Imaging 2002;18:41-51. [Crossref] [PubMed]
- Marshall BE, Hanson CW, Frasch F, et al. Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution. Intensive Care Med 1994;20:379-89. [Crossref] [PubMed]
- Luecke T, Pelosi P. Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care 2005;9:607-21. [Crossref] [PubMed]
- Klinger JR. Hemodynamics and positive end-expiratory pressure in critically ill patients. Crit Care Clin 1996;12:841-64. [Crossref] [PubMed]
- Fessler HE, Brower RG, Wise RA, et al. Effects of systolic and diastolic positive pleural pressure pulses with altered cardiac contractility. J Appl Physiol (1985) 1992;73:498-505. [Crossref] [PubMed]
- Tucker HJ, Murray JF. Effects of end-expiratory pressure on organ blood flow in normal and diseased dogs. J Appl Physiol 1973;34:573-7. [Crossref] [PubMed]
- Galvis AG, Benson DW. Spontaneous continuous positive airway pressure (CPAP) breathing in the management of acute pulmonary edema in infants. Clin Pediatr (Phila) 1973;12:265-9. [Crossref] [PubMed]
- Perel A, Williamson DC, Modell JH. Effectiveness of CPAP by mask for pulmonary edema associated with hypercarbia. Intensive Care Med 1983;9:17-9. [Crossref] [PubMed]
- Steier J, Lunt A, Hart N, et al. Observational study of the effect of obesity on lung volumes. Thorax 2014;69:752-9. [Crossref] [PubMed]
- Reilly CC, Ward K, Jolley CJ, et al. Neural respiratory drive, pulmonary mechanics and breathlessness in patients with cystic fibrosis. Thorax 2011;66:240-6. [Crossref] [PubMed]
- Jolley CJ, Luo YM, Steier J, et al. Neural respiratory drive in healthy subjects and in COPD. Eur Respir J 2009;33:289-97. [Crossref] [PubMed]
- Jolley CJ, Luo YM, Steier J, et al. Neural respiratory drive and breathlessness in COPD. Eur Respir J 2015;45:355-64. [Crossref] [PubMed]
- Xiao S, Bastianpillai J, Ratneswaran C, et al. Continuous Positive Airway Pressure and Breathlessness in Obese Patients with Obstructive Sleep Apnea: A Pilot Study. Sleep 2016;39:1201-10. [Crossref] [PubMed]
- Ratneswaran C, Pengo M, Xiao S, et al. The effect of continuous positive airway pressure titration on blood pressure in obese subjects. Eur Respir J 2015;46:PA1560.
- Onofri A, Patout M, Arbane G, et al. S56 Neural respiratory drive and cardiac function in patients with obesity-hypoventilation-syndrome following setup of non-invasive ventilation for hypercapnic respiratory failure. Thorax 2016;71:A33-4. [Crossref]
- Held M, Walthelm J, Baron S, et al. Functional impact of pulmonary hypertension due to hypoventilation and changes under noninvasive ventilation. Eur Respir J 2014;43:156-65. [Crossref] [PubMed]
- Smith TC, Marini JJ. Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol (1985) 1988;65:1488-99. [Crossref] [PubMed]
- Liesching T, Nelson DL, Cormier KL, et al. Randomized trial of bilevel versus continuous positive airway pressure for acute pulmonary edema. J Emerg Med 2014;46:130-40. [Crossref] [PubMed]
- Lenique F, Habis M, Lofaso F, et al. Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am J Respir Crit Care Med 1997;155:500-5. [Crossref] [PubMed]
- Pang D, Keenan SP, Cook DJ, et al. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest 1998;114:1185-92. [Crossref] [PubMed]
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med 1997;25:620-8. [Crossref] [PubMed]
- Acute heart failure: diagnosis and management | Guidance and guidelines | NICE.
- Krum H, Jelinek MV, Stewart S, et al. 2011 update to National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the prevention, detection and management of chronic heart failure in Australia, 2006. Med J Aust 2011;194:405-9. [PubMed]
- JCS Joint Working Group. Guidelines for treatment of acute heart failure (JCS 2011). Circ J 2013;77:2157-201. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure,Developed in Collaboration With the American College of Chest Physicians, Heart Rhythm Society and International Society for Heart and Lung Transplantation Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2013;128:e240-327. [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89. [Crossref] [PubMed]
- Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014;161:210-20. [Crossref] [PubMed]
- White DP. Sleep-related breathing disorder.2. Pathophysiology of obstructive sleep apnoea. Thorax 1995;50:797-804. [Crossref] [PubMed]
- Fava C, Montagnana M, Favaloro EJ, et al. Obstructive sleep apnea syndrome and cardiovascular diseases. Semin Thromb Hemost 2011;37:280-97. [Crossref] [PubMed]
- Jenner R, Fatureto-Borges F, Costa-Hong V, et al. Association of obstructive sleep apnea with arterial stiffness and nondipping blood pressure in patients with hypertension. J Clin Hypertens (Greenwich) 2017;19:910-8. [Crossref] [PubMed]
- Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 2003;177:385-90. [Crossref] [PubMed]
- Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol 2008;52:686-717. [Crossref] [PubMed]
- Fava C, Dorigoni S, Dalle Vedove F, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest 2014;145:762-71. [Crossref] [PubMed]
- Ali NJ, Davies RJ, Fleetham JA, et al. The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest 1992;101:1526-32. [Crossref] [PubMed]
- Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897-904. [Crossref] [PubMed]
- Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension 2000;35:144-7. [Crossref] [PubMed]
- Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J 2003;21:241-7. [Crossref] [PubMed]
- Dursunoğlu N, Dursunoğlu D, Cuhadaroğlu C, et al. Acute effects of automated continuous positive airway pressure on blood pressure in patients with sleep apnea and hypertension. Respiration 2005;72:150-5. [Crossref] [PubMed]
- Pengo MF, Ratneswaran C, Berry M, et al. Effect of Continuous Positive Airway Pressure on Blood Pressure Variability in Patients With Obstructive Sleep Apnea. J Clin Hypertens (Greenwich) 2016;18:1180-4. [Crossref] [PubMed]
- Bironneau V, Goupil F, Ducluzeau PH, et al. Association between obstructive sleep apnea severity and endothelial dysfunction in patients with type 2 diabetes. Cardiovascular Diabetology 2017;16:39. [Crossref] [PubMed]
- Schwarz EI, Puhan MA. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: A systematic review and meta-analysis. Respirology 2015;20:889-95. [Crossref] [PubMed]
- Ip MS, Tse HF, Lam B, et al. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 2004;169:348-53. [Crossref] [PubMed]
- Tulmaç M, Tireli E, Ebinç H, et al. Effect of overnight nasal continuous positive airway pressure treatment on the endothelial function in patients with obstructive sleep apnea. Anadolu Kardiyol Derg 2012;12:560-5. [PubMed]
- Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med 2000;162:2166-71. [Crossref] [PubMed]
- Chen WJ, Liaw SF, Lin CC, et al. Effect of Nasal CPAP on SIRT1 and Endothelial Function in Obstructive Sleep Apnea Syndrome. Lung 2015;193:1037-45. [Crossref] [PubMed]
- Noda A, Nakata S, Koike Y, et al. Continuous positive airway pressure improves daytime baroreflex sensitivity and nitric oxide production in patients with moderate to severe obstructive sleep apnea syndrome. Hypertens Res 2007;30:669-76. [Crossref] [PubMed]
- Doonan RJ, Scheffler P, Lalli M, et al. Increased arterial stiffness in obstructive sleep apnea: a systematic review. Hypertens Res 2011;34:23-32. [Crossref] [PubMed]
- Jelic S, Bartels MN, Mateika JH, et al. Arterial stiffness increases during obstructive sleep apneas. Sleep. 2002;25:850-5. [PubMed]
- Hoyos CM, Yee BJ, Wong KK, et al. Treatment of Sleep Apnea With CPAP Lowers Central and Peripheral Blood Pressure Independent of the Time-of-Day: A Randomized Controlled Study. Am J Hypertens 2015;28:1222-8. [Crossref] [PubMed]
- Jones A, Vennelle M, Connell M, et al. The effect of continuous positive airway pressure therapy on arterial stiffness and endothelial function in obstructive sleep apnea: a randomized controlled trial in patients without cardiovascular disease. Sleep Med 2013;14:1260-5. [Crossref] [PubMed]
- Korcarz CE, Benca R, Barnet JH, et al. Treatment of Obstructive Sleep Apnea in Young and Middle-Aged Adults: Effects of Positive Airway Pressure and Compliance on Arterial Stiffness, Endothelial Function, and Cardiac Hemodynamics. J Am Heart Assoc 2016;5:e002930. [PubMed]
- Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest 2005;128:2116-22. [Crossref] [PubMed]
- Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009;373:82-93. [Crossref] [PubMed]
- Yumino D, Redolfi S, Ruttanaumpawan P, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010;121:1598-605. [Crossref] [PubMed]
- Kent BD, Steier J. A Brief History of Fluid and Sleep. Am J Respir Crit Care Med 2015;191:1219-20. [Crossref] [PubMed]
- Stoohs R, Guilleminault C. Cardiovascular changes associated with obstructive sleep apnea syndrome. J Appl Physiol (1985) 1992;72:583-9. [Crossref] [PubMed]
- Cargill RI, Kiely DG, Lipworth BJ. Adverse effects of hypoxaemia on diastolic filling in humans. Clin Sci (Lond) 1995;89:165-9. [Crossref] [PubMed]
- Tkacova R, Rankin F, Fitzgerald FS, et al. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation 1998;98:2269-75. [Crossref] [PubMed]
- Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA 2015;314:2280-93. [Crossref] [PubMed]
- Guo J, Sun Y, Xue LJ, et al. Effect of CPAP therapy on cardiovascular events and mortality in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath 2016;20:965-74. [Crossref] [PubMed]
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014;32:2341-50. [Crossref] [PubMed]
- Liu L, Cao Q, Guo Z, et al. Continuous Positive Airway Pressure in Patients With Obstructive Sleep Apnea and Resistant Hypertension: A Meta-Analysis of Randomized Controlled Trials. J Clin Hypertens (Greenwich) 2016;18:153-8. [Crossref] [PubMed]
- Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, et al. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 2007;131:453-9. [Crossref] [PubMed]
- Lloberes P, Sampol G, Espinel E, et al. A randomized controlled study of CPAP effect on plasma aldosterone concentration in patients with resistant hypertension and obstructive sleep apnea. J Hypertens 2014;32:1650-7; discussion 7. [Crossref] [PubMed]
- de Souza F, Muxfeldt ES, Margallo V, et al. Effects of continuous positive airway pressure treatment on aldosterone excretion in patients with obstructive sleep apnoea and resistant hypertension: a randomized controlled trial. J Hypertens 2017;35:837-44. [Crossref] [PubMed]
- Lacedonia D, Tamisier R, Roche F, et al. Respective effects of OSA treatment and angiotensin receptor blocker on aldosterone in hypertensive OSA patients: a randomized cross-over controlled trial. Int J Cardiol 2014;177:629-31. [Crossref] [PubMed]
- Deng G, Qiu ZD, Li DY, et al. Effects of continuous positive airway pressure therapy on plasma aldosterone levels in patients with obstructive sleep apnea: A meta-analysis. J Huazhong Univ Sci Technolog Med Sci 2016;36:619-25. [Crossref] [PubMed]
- Ohike Y, Kozaki K, Iijima K, et al. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure--possible involvement of nitric oxide and asymmetric NG, NG-dimethylarginine. Circ J 2005;69:221-6. [Crossref] [PubMed]
- Panoutsopoulos A, Kallianos A, Kostopoulos K, et al. Effect of CPAP treatment on endothelial function and plasma CRP levels in patients with sleep apnea. Med Sci Monit 2012;18:CR747-51. [Crossref] [PubMed]
- de Lima AM, Franco CM, de Castro CM, et al. Effects of nasal continuous positive airway pressure treatment on oxidative stress and adiponectin levels in obese patients with obstructive sleep apnea. Respiration 2010;79:370-6. [Crossref] [PubMed]
- El Solh AA, Akinnusi ME, Baddoura FH, et al. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med 2007;175:1186-91. [Crossref] [PubMed]
- Xu H, Wang Y, Guan J, et al. Effect of CPAP on Endothelial Function in Subjects With Obstructive Sleep Apnea: A Meta-Analysis. Respir Care 2015;60:749-55. [Crossref] [PubMed]
- Kitahara Y, Hattori N, Yokoyama A, et al. Effect of CPAP on brachial-ankle pulse wave velocity in patients with OSAHS: an open-labelled study. Respir Med 2006;100:2160-9. [Crossref] [PubMed]
- Saito T, Saito T, Sugiyama S, et al. Effects of long-term treatment for obstructive sleep apnea on pulse wave velocity. Hypertens Res 2010;33:844-9. [Crossref] [PubMed]
- Vlachantoni IT, Dikaiakou E, Antonopoulos CN, et al. Effects of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea in arterial stiffness: a meta-analysis. Sleep Med Rev 2013;17:19-28. [Crossref] [PubMed]
- Kato M, Kumagai T, Naito R, et al. Change in cardio-ankle vascular index by long-term continuous positive airway pressure therapy for obstructive sleep apnea. J Cardiol 2011;58:74-82. [Crossref] [PubMed]
- Seetho IW, Asher R, Parker RJ, et al. Effect of CPAP on arterial stiffness in severely obese patients with obstructive sleep apnoea. Sleep Breath 2015;19:1155-65. [Crossref] [PubMed]
- Keles T, Durmaz T, Bayram NA, et al. Effect of continuous positive airway pressure therapy on aortic stiffness in patients with obstructive sleep apnea syndrome. Echocardiography 2009;26:1217-24. [Crossref] [PubMed]
- Tagetti A, Bonafini S, Zaffanello M, et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J Hypertens 2017;35:125-31. [Crossref] [PubMed]
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007;49:1625-31. [Crossref] [PubMed]
- Solin P, Roebuck T, Johns DP, et al. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med 2000;162:2194-200. [Crossref] [PubMed]
- Bradley TD, Floras JS. Sleep Apnea and Heart Failure. Part II: Central Sleep Apnea. Circulation 2003;107:1822-6. [Crossref] [PubMed]
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003;348:1233-41. [Crossref] [PubMed]
- Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004;169:361-6. [Crossref] [PubMed]
- Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005;353:2025-33. [Crossref] [PubMed]
- Sharma BK, Bakker JP, McSharry DG, et al. Adaptive servoventilation for treatment of sleep-disordered breathing in heart failure: a systematic review and meta-analysis. Chest 2012;142:1211-21. [Crossref] [PubMed]
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med 2015;373:1095-105. [Crossref] [PubMed]
- McEvoy RD, Thornton AT. Treatment of obstructive sleep apnea syndrome with nasal continuous positive airway pressure. Sleep 1984;7:313-25. [Crossref] [PubMed]
- McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:1108-14. [Crossref] [PubMed]
- Henderson LA, Fatouleh RH, Lundblad LC, et al. Effects of 12 Months Continuous Positive Airway Pressure on Sympathetic Activity Related Brainstem Function and Structure in Obstructive Sleep Apnea. Front Neurosci 2016;10:90. [Crossref] [PubMed]
- Granton JT, Naughton MT, Benard DC, et al. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med 1996;153:277-82. [Crossref] [PubMed]
- Johnson CB, Beanlands RS, Yoshinaga K, et al. Acute and chronic effects of continuous positive airway pressure therapy on left ventricular systolic and diastolic function in patients with obstructive sleep apnea and congestive heart failure. Can J Cardiol 2008;24:697-704. [Crossref] [PubMed]
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med 2016;375:919-31. [Crossref] [PubMed]
- Yu J, Zhou Z, McEvoy RD, et al. Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis. JAMA 2017;318:156-66. [Crossref] [PubMed]
- Abuzaid AS, Al Ashry HS, Elbadawi A, et al. Meta-Analysis of Cardiovascular Outcomes With Continuous Positive Airway Pressure Therapy in Patients With Obstructive Sleep Apnea. The American Journal of Cardiology. 2017;120:693-9. [Crossref] [PubMed]


