A novel suction-based lung-stabilizing device for video-assisted thoracoscopic surgical procedures
Introduction
Manipulation of the lung during video-assisted thoracoscopic surgery (VATS) can be complicated by the organ’s slippery surface and fragile tissue. Furthermore, surgeons must work within the tight confines of the rigid thoracic cage. During these procedures, surgeons usually push aside or stabilize the lung using conventional devices such as cotton-tipped medical applicators and graspers. However, these devices can damage the lung tissue, resulting in bleeding and air leakage. To address these problems, we developed a novel suction-based lung-stabilizing device, and demonstrated the prototype’s utility and safety using animal experiments (1).
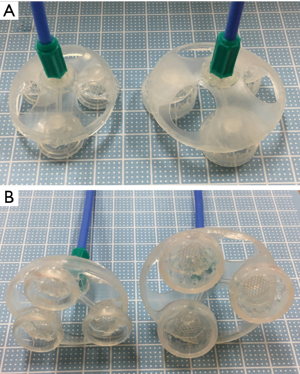
The device is equipped with three suction cups. In the center of each suction cup, there is a 2-mm vacuum port through which 1-mm-diameter tubes are inserted. As shown in Figure 1, the three tubes are gathered into a single duct at the top of the device. The device was connected to a suction system in the operating room to allow surgeons to stabilize and control the lung, and the negative pressure was set at −400 mmHg. Two sizes (small and large) of the device were developed to be used in different situations. The small-sized device uses 15-mm diameter cups, and the large-sized device uses 20-mm diameter cups (Figure 1). A 20-mm incision will allow the insertion of the novel device (of either size) if folded. This device was approved by Japanese Authorities for use as a management medical equipment (Class II) in September 2016.
The device was used in a clinical setting to successfully perform VATS right middle lobectomy (RML). Here, we report our experience with the clinical application of this lung-stabilizing device in a VATS lobectomy.
Surgical techniques
An asymptomatic 81-year-old man was admitted to our hospital with a diagnosis of lung cancer. The thoracic computed tomography scan revealed a space-occupying lesion located in the right middle lobe without enlargement of the mediastinal and hilar lymph nodes. Before surgery, no distant metastasis was detected. The preoperative diagnosis was primary lung cancer (cT1bN0M0, Stage IA) based on the 7th edition of the UICC TNM classification system. RML and mediastinal lymph dissection were selected as primary treatment.
An RML was performed using VATS through the following three ports: a 3-cm access port in the fourth intercostal space at the anterior axillary line, a 5-mm assist port in the seventh intercostal space at the scapular line, and a 5-mm camera port in the seventh intercostal space at the mid-axillary line.
After the incisions were made, the large-sized device was inserted into the thoracic cavity via the access port and connected to a suction system. As shown in the video, the device was guided to the appropriate position on the middle lobe’s surface by an assistant and controlled using an endoscopic grasper. This provided a sufficient field of view and appropriate tension to allow dissection on the target tissue. The device enabled retraction of the residual lobe to provide space for the surgical procedure to be carried out.
The target lung lobe was pulled freely to the ventral or dorsal side to allow operation on the pulmonary hilar structures. The device was able to firmly grasp the lung without detaching from the lung surface, and was easily disengaged by releasing the suction pressure when we needed to change the surgical field of view. A stapling device (Endo-GIATM purple 45 mm) was inserted to transect the middle lobe bronchus after dissection of the pulmonary artery and vein. Although one of the three suckers was not attached to the surface, sufficient traction was maintained. Pulling the subject lobe using the device provided an excellent field of view, and we were easily able to check the tip of the auto-suture device (Figure 2).

RML was safely performed without organ damage or difficulties during the surgery. No traumatic changes were observed on the surface of the lung upon which the device was applied. Total operation time was 90 minutes. The patient had an uneventful postoperative course.
Comments
VATS procedures have become increasingly common, and currently account for approximately 68% of all lobectomies conducted in Japan (3). However, the use of VATS is frequently accompanied by issues such as shapeless deflated lungs and an increased risk of organ injury. These problems may be due to a lack of specific devices for lung retraction that ensure an adequate view during surgery without damaging the organ. The use of suction-based devices for organ stabilization (e.g., OCTOPUS™ tissue stabilizer and the Starfish™ heart positioner) during cardiovascular surgery enables off-pump coronary artery bypass grafting to be performed efficiently without tissue damage (4-7). We hypothesized that a similar suction-based device would have applications for stabilizing lungs during VATS procedures, and developed a novel silicon-based device for this purpose. The utility and safety of the prototype were previously demonstrated using animal experiments (1). This report is the first to describe the clinical use of a suction-based device in VATS lobectomy. The device was able to stabilize the lobe and provide a good view for surgery. We designed the suction tubes to have thin 1-mm diameters to prevent the simultaneous release of negative pressure between the lung surface and suction cups even if one of the suction cups were to accidentally detach from the lung.
While conventional devices such as cotton-tipped medical applicators are only able to push the target lung lobe, the novel device was also able to pull up the lobe. In our opinion, this device would be particularly useful when applied to dissecting hilar structures because pulling up the target lobe would provide a space behind these structures, thereby allowing the safe and efficient encircling of vessels.
In this case presentation, no organ injury was observed during the VATS procedure. No macroscopic changes or traumatic damage were detected in the resected lung specimen or the residual lobe that was in contact with the device during the procedure.
A practical drawback is the skill needed to control and deliver the device to an appropriate location on the target lung lobe. It is therefore necessary for surgeons to first become used to handling the device, and it may be advantageous to implement further improvements for usability, such as modifying the shape of the gripping point or developing a specific endoscopic grasper. In addition, there is a need for further modification of the device (such as attaching a curved control stick) before it can be used in single-port VATS.
This novel lung-stabilizing device was found to have useful clinical applications in a VATS RML procedure.
Acknowledgements
We would like to express our appreciation to Messrs. Fumikazu Watanabe and Akihiro Asai of Fuji Systems Corporation (Tokyo, Japan) for supplying us with the device.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Muranishi Y, Sato T, Yutaka Y, et al. Development of a novel lung-stabilizing device for VATS procedures. Surg Endosc 2017;31:4260-7. [Crossref] [PubMed]
- Muranishi Y, Sato T, Ueda Y, et al. Use of the novel lung-stabilizing device during a video-assisted thoracoscopic surgical procedure. Asvide 2018;5:090. Available online: http://asvidett.amegroups.com/article/view/22888
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Masuda M, et al. Thoracic and cardiovascular surgery in Japan during 2013: annual report by the Japanese association for thoracic surgery. Gen Thorac Cardiovasc Surg 2015;63:670-701. [Crossref] [PubMed]
- Shimizu K, Nakano T, Kakegawa S, et al. Pericardium reconstruction with the starfish heart positioner after extended thymectomy with combined left side pericardium resection. Ann Thorac Surg 2012;94:2136-8. [Crossref] [PubMed]
- Matsuura N, Ishikawa S, Misaki N, et al. A new application for the heart positioner in operations for mediastinal tumors. Ann Thorac Surg 2010;90:2063-4. [Crossref] [PubMed]
- Gründeman PF, Budde R, Beck HM, et al. Endoscopic exposure and stabilization of posterior and inferior branches using the endo-starfish cardiac positioner and the endo-octopus stabilizer for closed-chest beating heart multivessel CABG: hemodynamic changes in the pig. Circulation 2003;108:II34-8. [Crossref] [PubMed]
- Gründeman PF, Verlaan CW, van Boven WJ, et al. Ninety-degree anterior cardiac displacement in off-pump coronary artery bypass grafting: the Starfish cardiac positioner preserves stroke volume and arterial pressure. Ann Thorac Surg 2004;78:679-84; discussion 684-5. [Crossref]


