Value of combining serum carcinoembryonic antigen and PET/CT in predicting EGFR mutation in non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related death in the world. About 85% of lung cancers are non-small cell lung cancers (NSCLC). The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein, which contributes to tumor cell proliferation, differentiation, angiogenesis and anti-apoptosis. Some studies had shown that EGFR mutations are more common in females, non-smokers, East Asians, and patients with adenocarcinoma (1,2). EGFR Tyrosine kinase inhibitors (EGFR-TKIs) had been proved to be more efficient than conventional chemotherapy in EGFR mutation-positive NSCLC patients (3,4). Meanwhile, EGFR-TKIs have little effect in patients with wild-type EGFR (5). Thus, clarifying patients’ EGFR mutation status is very important before anti-tumor therapy. However, sometimes it is hard to get enough tumor tissues for EGFR mutation testing, especially in advanced stage patients. Therefore, developing other noninvasive methods to predict EGFR mutation status is necessary. 18F-Fluoro-2-deoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) has been widely used in staging and evaluating the treatment effect of NSCLC. FDG uptake, usually using SUVmax of the primary tumor, can reflect tumor cell proliferation and glucose metabolism (6). Some studies have reported that SUVmax can be used to predict EGFR mutation status in NSCLC (7-11). However, there is no consensus conclusion. Thus more studies are needed to investigate the association between SUVmax and EGFR mutation status in NSCLC. Tumor markers are widely used to diagnose, monitor therapy response and recurrence in NSCLC (12,13). Carcinoembryonic antigen (CEA) is one of the most commonly used tumor markers in NSCLC. Some studies had shown that CEA is a significant prognostic predictor in patients treated with EGFR-TKIs (14-16). Some researchers also proposed that CEA level have some relation with EGFR mutation status (17). However, no consensus conclusions were reached.
Few studies had investigated the value of combining clinical features, pretreatment serum CEA level and SUVmax of the primary tumor in predicting EGFR mutation status in NSCLC. Thus, the purpose of this study is to analyze these clinical parameters in NSCLC and evaluate whether they can help predicting the EGFR mutation status in NSCLC.
Methods
Patients and inclusion criteria
This study was approved by our institutional review board (approval 2013-07 revision one). We retrospectively reviewed the medical records of all patients who were diagnosed NSCLC, underwent EGFR mutation test and 18F-FDG PET/CT scan less than one month before receiving any therapy between March 2011 and December 2014 at our hospital (the First Affiliated Hospital of Sun Yat-sen University). Patients’ characteristics were gathered by a chart, including age, gender, smoking status and pretreatment serum CEA level. Smoking status was defined as follows: never-smokers had smoked less than 100 cigarettes during their lifetime; current smokers were those who were still smoking or had quit smoking less than 1 year at the time of diagnosis; the remaining patients were categorized as former smokers. Pathological characteristics including tumor histology, grade and stage were collected. Patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual (18). We excluded five patients who had immeasurable lesions.
PET/CT scanning
PET/CT scans were performed with a Gemini GXL 16 scanner (Philips, Netherlands) in three-dimensional acquisition mode. All patients were required to fast for at least 6 h to make sure the blood glucose level was no more than 140 mg/dL before 18F-FDG injection. After i.v. injection of 5.18 MBq/kg 18F-FDG one hour later, imaging was obtained using a low-dose (120 kVp, 140 mA, 0.5 s per CT rotation, 5 mm collimation, 7.5 mm slice thickness, 1.25 mm pitch) two-slice CT scan from the head to the proximal thighs. Subsequently, PET images were acquired with a time of 3 min per bed position in 3-dimensional mode. After that, PET images were fused with the attenuation correction CT images to reconstruct PET/CT image using ordered-subset expectation maximization (OSEM) (4 iterations and 8 subsets). The final images were displayed by Xeleris Software (Philips, Netherlands).
PET data analysis
The FDG-PET data were analyzed by two experienced nuclear medicine physicians who were blind to the EGFR mutation status. Regions of interest (ROI) were placed on the primary tumors and mediastinal lymph nodes with abnormal FDG uptake on reconstructed PET/CT images. To minimize variation according to the size of ROIs, the maximum pixel activity within the ROI was recorded to calculate the SUVmax [SUVmax = maximum pixel activity/(injected dose/body weight)].
EGFR mutational analysis
Genomic DNA was extracted from tumor tissue using QIAamp DNA Formalin-fixed paraffin-embedded Tissue Kit (Qiagen. Hilden, Germany) following the manufacturer’s protocols. The DNA was diluted to 2–3 ng/µL in EGFR mutation test. The EGFR mutation detection kit (Amoy Diagnostics, Xiamen, China), which is based on the Amplified Refractory Mutation System (ARMS) technology, was used to identify the 29 most common types of EGFR mutations from exon 18 to 21. All experiments were performed following the manufacturer’s protocols. Briefly, ten nanograms genomic DNA was added to 45 µL PCR master mix containing PCR primers, fluorescent probes, Taq DNA polymerase and PCR buffer for each assay. The PCR cycling concluded 3 steps: firstly, 1 cycle of 95 °C for 5 min; secondly, 15 cycles of 95 °C for 25 s, 64 °C for 20 s, 72 °C for 20 s; thirdly, 31 cycles of 93 °C for 25 s, 60 °C for 35 s, 72 °C for 20 s. After 47 cycles of amplification, the fluorescent signal was collected from FAM and HEX channels. The results were analyzed following the manufacturer’s protocols.
Statistical analysis
Student’s t-test was used to analyze continuous variables. The results were expressed as the mean ± standard deviation (SD). Fisher’s exact test or Pearson’s chi-square test was used to analyzing categorical variables. A receiver operating characteristic (ROC) curve was generated to obtain the SUVmax and CEA cut-off values. The predictive value of the established criteria was assessed by calculating the area under the ROC curve (AUC). Multivariate logistic regression analysis was performed to test the associations between clinical factors and EGFR mutations. The multivariate logistic regression equation was used to predict a certain patient’s EGFR mutation status. A two-sided P value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software (version 20.0; SPSS, Chicago, IL).
Results
Patient characteristics and their associations with EGFR mutations
The baseline characteristics of patients are summarized in Table 1. In total, 210 patients (132 males and 78 females) were included in this study. Male (n=132; 62.9%), non-smoker (n=120; 57.1%) and advanced stage (III-IV) (n=152; 72.4%) accounted for the majority of the population. The median SUVmax of primary tumors was 8.5 (4.8–12.9). Among them, 70 patients (33.3%) were found to be EGFR mutation-positive, the rest were wild-type EGFR. The predominant mutation subtypes were the L858R point mutation in exon 21 (n=35; 16.7%) and the exon 19 deletion (n=31; 14.8%). The rest included L861Q point mutation in exon 21 (n=2; 1.0%), S768I missense mutation in exon 20 (n=1; 0.5%) and G719X point mutation in exon 18 (n=1; 0.5%). Univariate analysis was used to evaluate the association between clinical factors and EGFR mutation status. Results were summarized in Table 2. EGFR mutations were more frequent in females than males (57.7% vs. 18.9%; P<0.001), in never-smokers than smokers (47.5% vs. 14.4%; P<0.001), in patients with adenocarcinomas than non-adenocarcinomas (41.0% vs. 8.2%; P<0.001). Neither tumor grade (P=0.081) nor tumor size (P=0.316) showed relevance with EGFR mutation status.

Full table
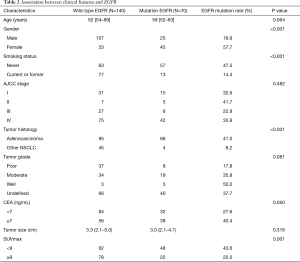
Full table
Association between SUVmax and EGFR mutations
The ROC curve revealed that the SUVmax cutoff point was 9.0, and the calculated AUC was 0.62 (95% CI, 0.54–0.70). According to the selected SUVmax cutoff point 9.0, which the sensitivity, specificity, positive and negative predictive values for predicting EGFR mutation were 70.0%, 54.3%, 43.3% and 78.4%, patients were divided into two groups: low SUVmax group (<9.0) and high SUVmax group (≥9.0). EGFR mutations were found more frequently in the low SUVmax group than in the high SUVmax group (43.6% vs. 22.0%; P=0.001).
Association between CEA and EGFR mutations
The ROC curve revealed that the CEA cutoff point was 7.0 ng/mL, and the calculated AUC was 0.56 (95% CI, 0.47–0.64). According to the selected CEA cutoff point 7.0 ng/mL, which the sensitivity, specificity, positive and negative predictive values for predicting EGFR mutation were 54.3%, 60.0%, 40.4% and 72.5%, patients were divided into two groups: low CEA group (<7.0 ng/mL) and high CEA group (≥7.0 ng/mL). EGFR mutations were found more frequently in high CEA group than in the low CEA group (40.4% vs. 27.6%; P=0.05).
Multivariate analysis
We included all variables with P<0.2 in univariate analysis, including SUVmax, gender, tumor histology, CEA, smoking status, age and tumor grade in the multivariate logistic regression analysis, despite smoking status (P=0.121), age (P=0.864) and tumor grade (P=0.364), the rest were statistically significant predictors for EGFR mutation (Table 3).

Full table
ROC curve analysis revealed that the combination of these four factors had a relatively high predictive value as the calculated AUC was 0.80 (95% CI, 0.74–0.86) (Figure 1). Using the cutoff point 0.3432, which was selected by maximum Youden’s index, the sensitivity and specificity for predicting EGFR mutations were 81.4% and 72.1%, respectively.
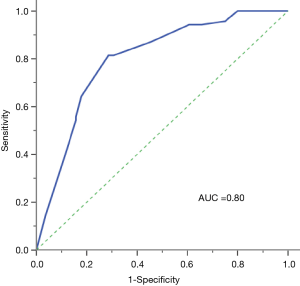
Multivariate logistic regression formulation was performed as follows: Y = ex/(1+ ex), X =−8.273+1.713 × gender +1.402 × histology +0.735 × CEA +0.921 × SUVmax. Compare patients’ calculated values with cutoff point 0.3432 (Table 4). If the value was higher than 0.3432 we concluded patient is EGFR mutation-positive, otherwise EGFR wild-type.
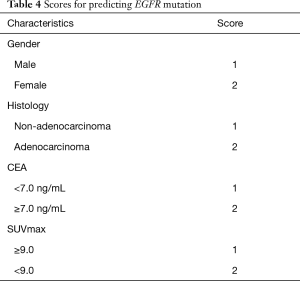
Full table
Discussion
Determination of the molecular profile has become standard practice in the management of patients with NSCLC. However, this assessment is frequently impaired by insufficient tumor tissue or technically deficiency (19). Therefore, alternative noninvasive strategies, such as 18F-FDG PET/CT, serum CEA, for predicting the mutation profile could help overcome these limitations and could be of value.
Our results show that 18F-FDG uptake is significantly increased in NSCLC tumors harboring EGFR mutations. We found that SUVmax lower than 9.0 was a significant predictor for EGFR mutations. Previous studies have shown contradictory results. Huang et al. indicated that patients with SUVmax higher than 9.5 were more likely to be EGFR mutation-positive (11). Study of Ko et al. was in accordance with Huang et al., they concluded that SUVmax higher than 6.0 was a significant predictor for EGFR mutations (9). The possible reason for the different results observed is that for acquiring further information in NSCLC patients, the histological type of our study cases was not only adenocarcinoma but also included non-adenocarcinoma (23.3%), which has been shown to have a different FDG uptake and distinct tumor biology. Conversely, Mak et al. reported that high FDG uptake value (normalized SUVmax >5) correlated with EGFR wild-type genotype in Western NSCLC patients (10). As their study included mostly white people 88% (88/100), their result may only represent Western people. Na et al. investigated 100 South Korean NSCLC patients, concluded that patients with low SUVmax (<9.2) was more likely to be EGFR mutation-positive (7). However, their study has a relatively small sample size (n=100), and EGFR mutation rate was only 21%, lower than common Asian populations. Our study included 210 NSCLC patients with stage I to IV and tumor histology which contains adenocarcinoma, squamous cell carcinoma, adenosquamous cell carcinoma and other subtypes of NSCLC, both smokers and nonsmokers, thus could be the better representative of NSCLC patients of the Asian population.
Our study found that patients with lower SUVmax were more likely to be EGFR mutation-positive, the reasons as follows: (I) In NSCLC, SUVmax differs in histology types: Squamous cell carcinoma always had a higher SUVmax than adenocarcinoma (20-23). A series of studies had confirmed that FDG uptake value had much to do with glucose transports (GLUTs). In NSCLC, GLUT1 is dominant in deciding FDG uptake value. Expression of GLUT1 in squamous cell carcinoma is much higher than adenocarcinoma (20,21), which would lead to an increase in FDG uptake. As EGFR mutations more frequently happen in adenocarcinoma than squamous cell carcinoma, the underlying reason that patients with lower SUVmax are more likely to be EGFR mutation-positive may be caused by histology difference. (II) This result maybe relates with hypoxia-inducible factor (HIF-1) protein. HIF-1 was shown to be related to regulating the genes responsible for increased utilization of glucose and energy metabolism (24). And cell lines with EGFR mutations expressed high basal levels of HIF-1α (25). 18F-FDG uptake is also shown to be associated with the presence of HIF-1 in other malignancies including cervix, cancer of the brain, the oral cavity and breast (24,26,27). However, some studies reported that there was no correlation or negative correlation between SUVmax and HIF-1 (24,28). (III) SUVmax also differs in subtypes of lung adenocarcinoma: Chiu et al. reported that GLUT1 expression and FDG uptake values were lower in terminal-respiratory-unit (TRU) type adenocarcinoma than non-TRU type (29). TRU-type adenocarcinoma is more likely to be EGFR mutation-positive (30). Besides, SUVmax of lepidic carcinomas, which is more common to be EGFR mutation-positive, is usually lower than other types of adenocarcinoma (31,32).
Currently, serum CEA is widely used in diagnosing and evaluating treatment in NSCLC, especially in adenocarcinoma. Some studies had reported that pretreatment serum CEA level could predict therapy effect of EGFR-TKIs in NSCLC. High level of pretreatment CEA indicates a good response to EGFR-TKIs and a better prognosis than normal CEA level (14-16). Shoji et al. first reported the relationship between serum CEA level and EGFR mutation in NSCLC (17). Their study pointed out that patients with higher serum CEA level at the time of recurrence were more likely to be EGFR mutation-positive than those with lower serum CEA level.
The mechanism of CEA predicting EGFR mutation status is not clear. Li et al. found that in NSCLC EGFR mutation had a positive correlation with CEA level in tumor tissue (r=0.237, P=0.003) (33). As both CEA and activation of EGFR signal pathway inhibits apoptosis, one possible hypothesis may be that elevated CEA level is caused by activation of anti-apoptosis signal pathway conducted by EGFR mutation (34,35). Our study is in accordance with Shoji et al., pointing out that EGFR mutation is more likely to happen in NSCLC patients with pretreatment serum CEA level higher than 7.0 ng/mL, predicting value of sensitivity and specificity were 54.3% and 60.0%, respectively.
Although previous studies have reported that variable FDG uptake, serum CEA levels are correlated with mutation status, respectively, one parameter alone is not sufficiently powerful and confident for predicting mutation status. The major strength of our study was that we established reliable clinical and imaging criteria for the prediction of EGFR mutation: high SUVmax and serum CEA levels. Our study combined pretreatment CEA level, SUVmax, gender and tumor histology in predicting EGFR mutation status, which is more efficient than a single factor like CEA level or SUVmax. The combining calculated AUC was 0.80, sensitivity and specificity for predicting EGFR mutation was 81.4% and 72.1%, while the calculated AUC of SUVmax and serum CEA were 0.62 and 0.56 respectively.
There are some limitations in our study: firstly, although the sample size of our study is larger than the other similar reported studies, it is still relatively small. Secondly, it is a retrospective study and needs prospective study to confirm our conclusion. Moreover, the mechanism in detail between SUVmax and EGFR mutation is still not clear. More basic experimental research is needed.
Conclusions
In conclusion, combining the use of pretreatment serum CEA level, SUVmax, gender and tumor histology is practical in predicting EGFR mutation status in NSCLC patients, especially when there isn’t enough tumor tissue or are unable to do EGFR mutation test. However, large multi-center prospective clinical trials are needed to confirm the current conclusion.
Acknowledgements
Funding: This work is supported by grants from the National Natural Science Foundation of China (81570008, Y Zhou), the Natural Science Foundation of Guangdong Province of China (2014A030313052, Y Zhou), and Science and Technology Program of Guangzhou, China (2014J4100132, Y Zhou).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This study was approved by our institutional review board (approval 2013-07 revision one, The First Affiliated Hospital of Sun Yat-sen University).
References
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Sequist LV, Bell DW, Lynch TJ, et al. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol 2007;25:587-95. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 2002;43:39-45. [PubMed]
- Na II, Byun BH, Kim KM, et al. 18F-FDG uptake and EGFR mutations in patients with non-small cell lung cancer: a single-institution retrospective analysis. Lung Cancer 2010;67:76-80. [Crossref] [PubMed]
- Caicedo C, Garcia-Velloso MJ, Lozano MD, et al. Role of [(1)(8)F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:2058-65. [Crossref] [PubMed]
- Ko KH, Hsu HH, Huang TW, et al. Value of F-18-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging 2014;41:1889-97. [Crossref] [PubMed]
- Mak RH, Digumarthy SR, Muzikansky A, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist 2011;16:319-26. [Crossref] [PubMed]
- Huang CT, Yen RF, Cheng MF, et al. Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol 2010;27:9-15. [Crossref] [PubMed]
- Cedres S, Nunez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer 2011;12:172-9. [Crossref] [PubMed]
- Sakao Y, Tomimitsu S, Takeda Y, et al. Carcinoembryonic antigen as a predictive factor for postoperative tumor relapse in early-stage lung adenocarcinoma. Eur J Cardiothorac Surg 2004;25:520-2. [Crossref] [PubMed]
- Okamoto T, Nakamura T, Ikeda J, et al. Serum carcinoembryonic antigen as a predictive marker for sensitivity to gefitinib in advanced non-small cell lung cancer. Eur J Cancer 2005;41:1286-90. [Crossref] [PubMed]
- Qin HF, Qu LL, Liu H, et al. Serum CEA Level Change and Its Significance Before and after Gefitinib Therapy on Patients with Advanced Non-small Cell Lung Cancer. Asian Pac J Cancer Prev 2013;14:4205-8. [Crossref] [PubMed]
- Chiu CH, Shih YN, Tsai CM, et al. Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer 2007;57:213-21. [Crossref] [PubMed]
- Shoji F, Yoshino I, Yano T, et al. Serum carcinoembryonic antigen level is associated with epidermal growth factor receptor mutations in recurrent lung adenocarcinomas. Cancer 2007;110:2793-8. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Lozano MD, Zulueta JJ, Echeveste JI, et al. Assessment of epidermal growth factor receptor and K-ras mutation status in cytological stained smears of non-small cell lung cancer patients: correlation with clinical outcomes. Oncologist 2011;16:877-85. [Crossref] [PubMed]
- Suzawa N, Ito M, Qiao S, et al. Assessment of factors influencing FDG uptake in non-small cell lung cancer on PET/CT by investigating histological differences in expression of glucose transporters 1 and 3 and tumour size. Lung Cancer 2011;72:191-8. [Crossref] [PubMed]
- Brown RS, Leung JY, Kison PV, et al. Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer. J Nucl Med 1999;40:556-65. [PubMed]
- Wang Y, Ma S, Dong M, et al. Evaluation of the factors affecting the maximum standardized uptake value of metastatic lymph nodes in different histological types of non-small cell lung cancer on PET-CT. BMC Pulm Med 2015;15:20. [Crossref] [PubMed]
- Zhang J, Chen L, Chen Y, et al. Tumor vascularity and glucose metabolism correlated in adenocarcinoma, but not in squamous cell carcinoma of the lung. PLoS One 2014;9:e91649. [Crossref] [PubMed]
- Cerci SS, Yalcin Y, Bozkurt KK, et al. Hypoxia-inducible factor-1alpha, adrenomedullin and Bcl-2 although expected are not related to increased uptake of fluorine-18-fluorodeoxyglucose in endometrial cancer. Hell J Nucl Med 2015;18:228-32. [PubMed]
- Lee JG, Wu R. Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1alpha in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia 2015;17:190-200. [Crossref] [PubMed]
- Park SI, Suh DS, Kim SJ, et al. Correlation between biological marker expression and F-fluorodeoxyglucose uptake in cervical cancer measured by positron emission tomography. Onkologie 2013;36:169-74. [Crossref] [PubMed]
- Bos R, van Der Hoeven JJ, van Der Wall E, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 2002;20:379-87. [Crossref] [PubMed]
- Xu H, Li B, Yu W, et al. Correlation between (1)(8)F-FDG uptake and the expression of glucose transporter-1 and hypoxia-inducible factor-1alpha in transplanted VX2 tumors. Nucl Med Commun 2013;34:953-8. [Crossref] [PubMed]
- Chiu CH, Yeh YC, Lin KH, et al. Histological subtypes of lung adenocarcinoma have differential (1)(8)F-fluorodeoxyglucose uptakes on the positron emission tomography/computed tomography scan. J Thorac Oncol 2011;6:1697-703. [Crossref] [PubMed]
- Yatabe Y, Kosaka T, Takahashi T, et al. EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. American Journal of Surgical Pathology 2005;29:633-9. [Crossref] [PubMed]
- Hsieh RK, Lim KH, Kuo HT, et al. Female sex and bronchioloalveolar pathologic subtype predict EGFR mutations in non-small cell lung cancer. Chest 2005;128:317-21. [Crossref] [PubMed]
- Haneda H, Sasaki H, Lindeman N, et al. A correlation between EGFR gene mutation status and bronchioloalveolar carcinoma features in Japanese patients with adenocarcinoma. Jpn J Clin Oncol 2006;36:69-75. [Crossref] [PubMed]
- Li Y. The feasibility of CEA to indicate EGFR gene mutations in non-small cell lung cancer. Unpublished MD dissertation, Dalian Medical University 2010:26-8.
- Cappuzzo F, Magrini E, Ceresoli GL, et al. Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 2004;96:1133-41. [Crossref] [PubMed]
- Ordonez C, Screaton RA, Ilantzis C, et al. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 2000;60:3419-24. [PubMed]


