Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials
Introduction
Chronic obstructive pulmonary disease (COPD) is a major chronic respiratory disease affecting over 380 million people worldwide (1). It has been estimated that COPD will be the third highest cause of global death by 2020 (2,3). Lung parenchymal damage (emphysema) and severe airflow obstruction are the typical pathophysiological features of COPD (4,5). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defined the stages of COPD based upon the severity of airflow obstruction by spirometry. In patients with forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) <0.70, for FEV1 ≥80% of the predicted value, the stage of these patients is defined as mild. If FEV1 ≥50% and <80% of the predicted value, the stage is defined as moderate. If FEV1 ≥30% and <50% of the predicted value, the stage is defined as severe. If FEV1 <30% of the predicted value, the stage is defined as very severe (4).
The pathological changes in COPD include chronic inflammation in the respiratory system and alveolar structural degeneration or destruction (4,6). Chronic inflammation was shown as a high count of inflammatory cell types like neutrophils in sputum and bronchoalveolar lavage (BAL) fluid (7), and macrophages in lung parenchyma (8). Increased neutrophils in sputum was associated with greater airflow obstruction in advanced COPD patients (9). Neutrophils and macrophages can release inflammatory mediators (such as cytokines and oxygen radicals) and enzymes (like protease) in the airways and lung parenchyma (10). Interleukin (IL)-1β is one major cytokine which involves in initiation and persistence of inflammation (11). Sputum neutrophils and alveolar macrophages from smokers can produce more IL-1β than those from non-smokers (12). IL-1β chronically produced in the respiratory system of mice caused lung inflammation and enlargement of airspaces (13). Protease like matrix metalloproteinases (MMPs) can mediate destruction of elastin which is the connective structure in lung parenchyma, and lead to airspace enlargement (14,15). Oxidative stress in COPD may amplify inflammatory response, impair the function of protease inhibitor like secretory leukocyte protease inhibitor (SLPI), and enhance elastin-breakdown by protease in lung parenchyma (16). In sum, inflammatory mediators together with enzymes which were produced by inflammatory cells caused the pathological changes in COPD (17).
Substantial work has been done to investigate the mechanisms of COPD and to find new drugs for treating COPD patients. Current treatments of COPD are aimed mainly at relieving the symptoms of patients, reducing the rate of exacerbations and improve the activity of the patients. The current mainstream medication for COPD are bronchodilators, which are either used on their own or in combination with inhaled corticosteroids. These bronchodilators transiently relieve the symptoms of dyspnea; however, they do not ameliorate the deterioration in lung function (6). According to the “Towards a Revolution in COPD Health” (TORCH) phase III clinical trial, the combined application of bronchodilators, including beta2-agonists and glucocorticosteroids, slightly improved the function of the lung (FEV1 increased by 0.092 liter) in moderate-to-severe COPD patients (18). The recently reported phase III clinical trial “TRILOGY” was designed to combine corticosteroids, muscarinic antagonists and beta2-agonists in a single inhaler for moderate-to-severe COPD patients. This therapy was reported to improve the FEV1 by 0.081 liter (19). To sum up, no drug is yet available that can stop the deterioration of functional structures and improve significantly the function of respiratory system in COPD patients (4).
In view of the limitation of these available drugs for treating COPD, there has been great interest in developing novel regenerative therapies with the aim of improving the function of the respiratory system by repairing or replacing the damaged pulmonary structures such as terminal bronchioles and alveoli seen in emphysematous patients.
Alveoli in the lung are responsible for gas exchange. Harmful particles from cigarette smoke (CS) or polluted air can reach alveoli via the airways. These particles can cause damage to types I and II epithelial cells of the alveoli (AEC I and AEC II), leading to airspace destruction, leading to the emphysema component of COPD (20). Stem cells with the ability to differentiate into lung cell types such as alveolar epithelial cells have the potential to be used to replace damaged or missing functional structures of the lung. Thus, regenerative therapeutic methods have been initiated to repair or replace the alveolar epithelial cells.
Regenerative approaches
The regenerative approaches have included extrinsic cell therapy such as the infusion of exogenous stem cells to repair the damaged structure of the respiratory system and intrinsic cell therapy such as the administration of small molecules to stimulate the endogenous lung stem/progenitor cells for regeneration and replacement of the damaged structures) (21). This is the first comprehensive review aimed to discuss more effective stem cell treatment solutions to COPD based on the experience learnt from current completed pre-clinical studies and clinical trials.
Extrinsic cell therapy
Various exogenous stem cells have been investigated for treating COPD in COPD animal models. These stem cells have included embryonic stem cells (ESCs), induced pluripotent stem (iPS) cells, mesenchymal stem cells (MSCs), and lung stem cells (LSCs).
The studies reported that both mouse- and human-derived ESCs could be differentiated into AEC II-like cells in vitro. The AEC II-like cells expressed surfactant proteins, such as the surfactant protein C (SPC), a marker of AEC II cells (22-25). AEC II-like cells (5×105) derived from human ESC lines were intratracheally infused into immunodeficient SCID/C57BL/6 mice that were administered with bleomycin (BLM) to induce acute pulmonary injury at 1 or 2 days after BLM-challenge. Control mice were treated with normal saline. At 10 days after dosing, treatment of AEC II-like cells significantly reduced alveolar structural damage with less cellular infiltration, decreased interstitial thickening, and lower collagen deposition in the lung sections compared with that of the control mice (26).
Ameliorative effects to the damaged lung were also demonstrated by transplanting AEC II-like cells (5×105, i.t.) that were derived from a mouse iPS cell line to C57BL/6 mice after the administration of BLM. At 12 days after transplantation, the mice treated with AEC II-like cells showed reduced extent of fibrosis shown as lower collagen deposition, and recovered lung tissue structure such as more organized epithelium, decreased interstitial thickening, less cystic air spaces, compared with that of the mice treated with normal saline. Besides, treatment of AEC II-like cells significantly reduced inflammatory cytokines including TNF-α and IL-6 measured in BAL fluid indicating anti-inflammatory effects of AEC II-like cells (27). However, despite the two successful pre-clinical studies mentioned above showing the potential of regenerating lung structures, there are still no clinical trials reported or registered applying cells derived from ESCs or iPS cells, or ESCs, or iPS cells to treat COPD patients (28). Reasons to non-application of these cell types include: ESCs have potentials for forming teratoma and immune rejection, and there are ethical concerns for application of ESCs (29,30); iPS cells can also form teratoma, and the current technique cannot produce reliable amount of clinical-grade iPS cells (30,31).
Therapeutic methods using MSCs to cure COPD have been extensively investigated. In addition to MSCs, the c-kit positive LSCs might be a new candidate product for curing COPD. MSCs and LSCs are now discussed in more detail.
MSCs
Pre-clinical results with the infusion of MSCs
MSCs are a population of multipotent cells that can differentiate into varieties of cell types including osteoblasts, chondrocytes, and adipocytes (32,33). Other features of MSCs are their plasticity and adherence. MSCs express surface markers, including CD73, CD90 and CD105. MSCs do not express markers of the hematopoietic lineage, including CD45, CD34, CD11c, CD14, CD19, CD79a and HLA-DR (34,35). Given the feature of low immunogenicity, both autologous and allogeneic MSCs derived from bone marrow or adipose (BM-MSCs and AD-MSCs) have been widely applied in pre-clinical studies. It was reported that animal recipients of allogenic MSCs tolerated well of these cells administered by injection (36,37).
It has been reported that BM-MSCs can be induced to differentiate into AEC II-like cells in vitro. Expression of pro-SPC and SPC (both at gene and protein levels) were detected after co-culture of human- or mouse-derived BM-MSCs with lung epithelial cells in modified small airway growth medium for 10–15 days (38-40). In addition, BM-MSCs can also be differentiated into AEC II-like cells in vivo (41-43). In one study, BrdU labeled BM-MSCs (2.5×106, i.v.) derived from a male rat were infused into female rats at days 0 and 7 after administration of BLM. At days 7, 14, and 28 after transplantation of BM-MSCs, the Y chromosome-specific SRY gene was detected as expressed in the lungs of the female rats. This suggested that the exogenous BM-MSCs had migrated to the recipients’ lung. Furthermore, at the three time points, the BrdU and SPC double-positive cells in the lung were observed. These observations not only confirmed that the BM-MSCs had migrated to the lung, but also indicated that the BM-MSCs had differentiated into AEC II-like cells (41).
The engraftment and survival rate of exogenous MSCs were reported as very low in COPD models (44,45). In one study, Quantum Dot (QD) 800-labeled AD-MSCs (5×105, i.v.) which was derived from human were injected into C57BL/6 mice of porcine pancreatic elastase (PPE) induced pulmonary emphysema model at day 7. By using Alu (a human specific gene)-based real time PCR method to determine the number of transplanted AD-MSCs, the authors detected only about 3,500 (0.7% of total transplanted cells) human AD-MSCs in the lung tissue samples at 1 hour after transplantation. The engraftment rate in the lung tissue samples decreased to around 0.4% and 0.06%, at 4 and 24 hours after injection respectively, and disappeared at 72 hours. In vivo imaging confirmed that the signals of QD representing the transplanted QD labelled AD-MSCs disappeared in the lung at 24 hours after transplantation (45). So, the low engraftment rate of exogenous MSCs in animal models is consistent throughout all studies; while the in vivo survival time varied among studies. And there was one study showed that exogenous MSCs survived as long as 8 weeks after transplantation into CS induced COPD rats (46). Despite the low engrafting rate of exogenous MSCs, most pre-clinical studies shown below have reported structural repair and functional restoration in COPD models after MSC infusion.
Infusion of allogeneic GFP+BM-MSCs (5×105, i.t.) derived from C57BL/6 GFP-transgenic mice into C57BL/6 wildtype mice of PPE-induced emphysema model at 14 days after PPE challenge, resulted in very low rate of engraftment into the injured lung tissue (shown as few GFP+ cells observed in the lung tissue sections under confocal microscope at day 7, and even fewer at days 14 and 21 after transplantation). Besides, few GFP and aquaporin 5 double-positive cells representing AEC II-like cells were observed in the lung tissue sections under confocal microscope at day 7 after transplantation, while no AEC II-like cell (shown as either GFP+/aquaporin 5+ cells or GFP+/pro-SPC+ cells) were detected at days 14 and 21. However, BM-MSCs treatment resulted in ameliorated alveolar structure of the mouse lungs at 7, 14, and 21 days after transplantation compared with that of the control group which was treated with PBS. And the level of pro-inflammatory cytokine IL-1β decreased in both BAL and lung samples at days 3 and 5 after BM-MSCs treatment. Thus, lung inflammation and airspaces enlargement caused by IL-1β were both reduced. The growth factors including hepatocyte growth factor (HGF) and epidermal growth factor (EGF), and protection factor for epithelial cells SLPI increased in the lung tissue at days 3 and 4 after infusion of BM-MSCs (47). HGF is a pleiotropic factor which can enhance cellular survival and proliferation (48,49). It was reported that HGF stimulated AEC II cell proliferation and induced lung tissue regeneration in COPD models (50,51). EGF was reported necessary for mouse epithelial cell proliferation and repair (52). SLPI can inhibit protease and consequently reduce the level of elastin-breakdown by protease in lung parenchyma (16). This work indicated that the exogenous allogeneic BM-MSCs repaired lung structures by secreting multiple paracrine factors that can reduce inflammation, promote regeneration and repair.
Another study applied [3H]-thymidine labelled allogeneic AD-MSCs (2.5×106, i.v.) derived from SD rats into other SD rats of PPE induced emphysema model at day 7 after PPE challenge. About 1.9% of the transplanted [3H]-thymidine+ AD-MSCs were found in the lung tissue of the recipient rats 3 hours after transplantation, while this ratio dramatically decreased 24 and 48 hours later. Pulmonary arterial gas exchange tests showed that the levels of resting PaO2 and A-aDO2 were significantly changed and restored to normal ranges at days 7 and 14 after transplantation of AD-MSCs compared with that of the normal saline treated rats, indicating allogeneic AD-MSCs restored the arterial oxygen tension and alveolar-arterial oxygen tension to normal levels. Treatment of AD-MSCs also significantly reduced the alveolar airspaces at days 7 and 14 after transplantation, suggesting structural repairs by AD-MSCs. Additionally, treatment of AD-MSCs also significantly increased the levels of HGF and cytokine-induced neutrophil chemoattractant 1 (CINC-1). All this evidence indicated that the allogeneic AD-MSCs may contribute to lung structural repair through anti-inflammatory pathways (53).
More pre-clinical studies have reported structural repair and functional restoration of damaged lung by exogenous MSCs in COPD models (46,54-58). Representative pre-clinical studies applying MSCs in COPD models are listed in Table 1. These pre-clinical studies applied either allogeneic BM-MSCs or AD-MSCs to different COPD models which were induced by either CS or PPE in different strains of mice or rats. Dose of cells, dosing frequency, and dosing methods were varied among the studies. Despite these differences, all of the studies presented positive outcomes at weeks or months after transplantation of stem cells. The positive outcomes were mostly shown as reduced inflammation and decreased airspace enlargement.
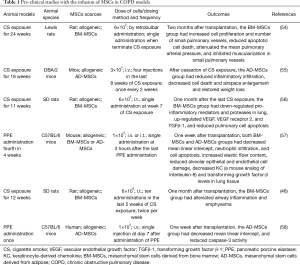
Full table
Except BM-MSCs and AD-MSCs, the lung-derived MSCs (lung-MSCs) were also investigated in COPD models. Autologous lung-MSCs were delivered into ovine COPD model. The delivered lung-MSCs were detected in the alveolar septum and peribronchiolar interstitium. Besides, after transplantation of lung-MSCs, the ovine had increased tissue mass and lung perfusion representing possible regeneration of lung tissue and recovery of lung function. This study suggested that exogenous autologous lung-MSCs have the potential to regenerate lung structure and induce lung functional recovery in COPD (59).
In summary, the repair effects of MSCs to the lung in COPD models was mainly caused by multiple paracrine factors secreted by exogenous MSCs. These factors can reduce inflammation, induce immunomodulation, improve the permeability of endothelial and epithelial cells, and facilitate tissue repair. Even the few regenerated AEC II-like cells from the limited engrafted exogenous MSCs may also have contributed to functional restoration by replacing the damaged structures (60-68). Each of these pre-clinical studies has indicated the potential efficacy of MSCs in COPD patients.
Clinical results with the infusion of MSCs
A pioneering multicenter, double-blind, and placebo-controlled phase II clinical trial (NCT00683722) applying MSCs in moderate-to-severe COPD patients was performed between 2008 and 2010 (69). In this trial, 30 of the 62 enrolled patients were treated with allogeneic BM-MSCs (Prochymal, Osiris Therapeutics Inc.; 1×108 cells per infusion, i.v. GTT, infused monthly for 4 months). The other 32 patients were treated with a placebo. Over the subsequent 2 years, no toxicities were detected in response to the exogenous BM-MSCs, and no functional changes were found after the infusion of BM-MSCs. Only at 1 month after the first infusion, the C-reactive protein (CRP) level mildly decreased compared with its level before the infusion.
The second completed clinical trial (NCT01110252) applied autologous bone marrow mononuclear cells (BMMCs) in COPD patients and was performed between 2009 and 2011 (70,71). BMMCs are heterogeneous cells that include lymphocytes, monocytes, hematopoietic progenitor cells (HPCs), and MSCs (72). Four patients with severe COPD were recruited. Their bone marrows were harvested separately and processed to separate out mononuclear cells—BMMCs; these fresh cells were infused back into the patients (1×108 cells/kg, i.v. GTT, single infusion). No side effects were reported related to transplantation of BMMCs. Pulmonary functional tests were performed over the next 2 years. Two of the four patients had a satisfactory performance with an increased FEV1 compared to the predicted values, suggesting a decrease in air trapping and an increase in elasticity. The authors noted that the relatively improved pulmonary function may be due to the anti-inflammation effects based on reported pre-clinical investigations and the first reported clinical trial (NCT00683722).
The third completed clinical trial (NCT01306513) was an open-label, non-randomized, non-blind, and prospective phase I clinical trial. This trial was performed between 2010 and 2012 (73). Bone marrows were collected from seven severe-to-very severe COPD patients while having the first lung volume reduction surgery (LVRS), and were cultured ex vivo to obtain BM-MSCs. Two infusions of autologous BM-MSCs were given to the patients (1–2×106 cells/kg, i.v.) firstly at 6–10 weeks after LVRS, secondly at 1 week later after the first infusion. All patients had a second LVRS at 3 weeks after the second infusion of BM-MSCs. No toxicity was observed related to infusions of BM-MSCs. One year after the second LVRS, the FEV1 and body weight increased significantly, lung densitometry also changed significantly among all patients compared with that before the first LVRS. But these changes were comparable to previous reports of patients only had LVRS, indicating the improved pulmonary structures, function, and body weight resulted from LVRS rather than BM-MSCs. The number of AEC II cells in the alveolar septa of all patients did not change according to histological staining and gene expression of SPC. The expression of CD31 (a marker of endothelial cells, by IHC staining) had 3-fold increase in the alveolar septa of the emphysematous lung tissue of all patients after treatment of LVRS and BM-MSCs. But whether the increased number of CD31+ cells represented enlarged population of endothelial cells remained to be investigated since the expression of CD31 was not restricted to endothelial cells. Besides, function of the increased CD31+ cells remained unknown. In summary, this study showed the safety and feasibility of autologous BM-MSCs in severe and very severe COPD patients. Because no control group was included, it cannot be concluded whether the changes of FEV1, body weight, lung densitometry and expression of CD31 were affected by LVRS or/and BM-MSCs.
Information on the three completed clinical trials that applied MSCs in COPD patients is given in Table 2.

Full table
Indications from the reported clinical trials of MSCs in COPD
The successful pre-clinical studies provided a significant basis for further clinical investigations with various sources of MSCs in COPD patients. However, given that the few preliminary clinical trials reported have been unsuccessful, this has weakened the potential for applying MSCs in COPD patients. Further clinical trials designed to use BM-MSCs or AD-MSCs to treat COPD have recently been registered, but these have not yet been started.
The advanced stage of the patients studied may be a major cause of the failure. The three currently reported clinical trials recruited moderate, severe or very severe COPD patients. The authors of the first clinical trial (NCT00683722) stated that the COPD stages of the patients may be the major cause of failure. They also noted that the damage to the pulmonary structures might be too severe to be reversed by the few transplanted MSCs (69). The widely used COPD models in pre-clinical studies only mimic the mild stage or, at most, the moderate stage of COPD patients, which may explain why pre-clinical studies all had structural repair and functional improvement after transplantation of MSCs. Interestingly, it was reported that the animals with stronger emphysema (induced by two stimuluses) recruited and engrafted more exogenous MSCs in the lung, than those engrafted in the lung with weaker emphysema (induced by one stimulus) (74). So, we may predict that patients with advanced stage of COPD might also recruit and engraft more exogenous MSCs in the lung, therefore should lead to structural repair and functional recovery as shown in animal models. However, the completed clinical trials did not meet our prediction. Nevertheless, this reminds us further research can be done to investigate how to enhance the engraftment of exogenous MSCs in the damaged lung. In addition, with the features of anti-inflammatory and immunomodulatory, exogenous MSCs may be more effective in acute lung disease than in chronic, progressive disease with severe structural damages.
Status of the exogenous MSCs was also key to the success or failure of trials. The allogeneic MSCs used in the first clinical trial (NCT00683722) were directly infused at the bedside immediately after thawing from frozen bags. This industrial process makes transplantation easy and convenient. However, the data indicated that newly thawed MSCs had lost part of their immunomodulatory capabilities (75). Failure of the first clinical trial (NCT00683722) and the third clinical trial (NCT01306513) may be both related to application of newly-thawed cells. Therefore, future trial designs should use fresh MSCs or cultured MSCs approximately 24 hours after thawing. The second clinical trial (NCT01110252) used fresh autologous BMMCs, and did not show significant improvement. It may be questioned whether the MSCs and HPCs within the infused BMMCs population from COPD patients have normal function. It has been reported that autologous BM-MSCs derived from COPD patients have similar phenotypes and functions as those from healthy people (76), leading to the thought that autologous BM-MSCs might not be a problem in this trial. However, it was known that BM-MSCs isolated from aging animals have shorter telomere length, decreased differentiation potential, impaired proliferation in vitro, and reduced potential to secrete paracrine factors in animals compared with that from younger animals; while the genotype and phenotype of AD-MSCs were almost the same as from younger and aging animals (77-79). Besides, aging mice derived BM-MSCs showed lower expression of certain cytokine and chemokine receptors, and they were less activated and mobilized to the site of lung injury than BM-MSCs derived from younger mice (80). Aging human derived BM-MSCs were also proven to have higher expressing of senescence related gene, shorter telomere length, lower proliferation and differentiation potential in vitro compared with that from younger donors (81). Function of HPCs were also reported to decline during aging (82-84). Considering the aging status of the four patients (59–76 years old) in the second trial, both the BM-MSCs and HPCs within the BMMCs population isolated from the aging patients might have lost part of the regenerative potential, thus potentially leading to the failure of the study. If this hypothesis is true, future clinical trials should apply autologous AD-MSCs when treating aging COPD patients or just use allogeneic MSCs from younger donors.
In addition to stage of COPD, status of MSCs (fresh or freezing, from younger or aging donors), other important parameters include doses of cells, dosing approaches, frequencies of dosing, and the design of study endpoints, age and scale of patient cases, inclusion of proper control groups. These different parameters may together have contributed to the conflicting results seen between the pre-clinical and clinical studies.
Human LSCs (hLSCs)
Pre-clinical results with the infusion of hLSCs
A population of c-kit positive cells with stem cell phenotypes (self-renewing, clonogenic, and multipotent) has been reported in the lungs of adult humans. These cells were named hLSCs (85).
The c-kit receptor is a cytokine receptor that was first found in murine hematopoietic stem cells (HSCs) (86,87). The c-kit was reported to be expressed on the surface of hLSCs and human cardiac stem cells (hCSCs) (85,88,89). The c-kit positive hLSCs do not express markers of HSCs-lineages (CD34, CD45, and CD133) or markers of MSCs-lineages (CD44, CD90, and CD105). Additionally, hLSCs do not express markers of the pulmonary lineage (85).
The hLSCs were demonstrated to be multipotent in vitro and in vivo. The c-kit positive and lineage negative hLSCs expressed markers of epithelial cells (TTF1, p63, pan-CK, CK5, SPC), endothelial cells (Ets1, vWf), and smooth muscle cells (SMCs) (GATA6, α-SMA) after exposure to dexamethasone in vitro. To demonstrate the multipotency of hLSCs in vivo, the EGFP-positive hLSCs (2×104 cells/injection, for six injections) were directly injected into the region in proximity to the CI lungs of mice shortly after CI injury when chest was opened. Two days later, approximately 30% of the EGFP-positive hLSCs were present at the CI region and its border. Ten to 14 days after injection, (EGFP and pro-SPC or SPC double-positive) human alveoli, (EGFP and pan-CK double-positive) human bronchioles, (EGFP and α-SMA or vWf double-positive) human SMCs or human endothelial cells were observed in the CI region of the recipient mice (85).
Indications from the reported application of c-kit positive hLSCs
The identified hLSCs can differentiate into both structures of endodermal origin (epithelial cells) and mesodermal origin (vessels) in injured mouse lungs, indicating a critical role of hLSCs in lung homeostasis and structural regeneration in lung injury. As described above, transplanted MSCs have relatively limited differentiation potential to gas-exchange units in the lung (only few AEC II-like alveoli cells were observed in most pre-clinical studies), and have very low integration rate, while the hLSCs showed advantage of more integrative and replacement potential. Furthermore, hLSCs have been shown to express Nanog, Oct3/4, Sox2 and Klf4, indicating pluripotency which also supported its endodermal and mesodermal differentiation potential. A very small subpopulation of embryonic-like stem cells among MSCs cultures showed lower expression of Oct4 in one study (90). Comparatively, hLSCs may have higher regeneration capability than MSCs. This in vivo study only suggested exogenous hLSCs may promote structural regeneration in cryo-induced lung injury. As to COPD, mechanisms of pathogenesis to which are heterogeneous, thus, we cannot predict hLSCs can also induce structural regeneration in COPD. So, more pre-clinical studies should be done to confirm that hLSCs can regenerate the injured lung tissue in different COPD animal models before being applied in clinical trials.
Intrinsic cell therapy
Activation of endogenous stem cells or progenitor cells to induce regeneration of the functional alveolar epithelial cells theoretically can be an alternative approach for reversing COPD. This regenerative approach was named “intrinsic cell therapy” based on the mobilization of endogenous stem cells or progenitor cells, an approach that differs from extrinsic cell therapy (21).
Previously, identified stem/progenitor cells in human respiratory system include basal cells, Clara cells, lung-MSCs and c-kit positive LSCs in the airways as well as AEC II cells in the alveoli (91-94). It has been reported that the basal cells and AEC II cells can re-enter the cell cycle from a quiescent state; they can multiply and promote tissue repair after lung injury (91). Research into activating these stem/progenitor cells remains at an earlier investigational stage and has not yet been fully approved and widely applied in pre-clinical and clinical studies.
Pre-clinical results after administering all trans-retinoic acids (ATRA) to activate the endogenous stem/progenitor cells
Various molecules (those vital to the development of the lung) have been investigated to determine whether they can activate the endogenous stem/progenitor cells in the lung. Regenerative effects have been tested after administering these molecules in COPD models.
Retinoic acid which is a derivative of vitamin A is required for the development of organs, including the lungs (95). Administration of ATRA (500 µg/kg, i.p., single injection) to SD rats that were pre-treated with dexamethasone was reported to have significantly reduced the volume density of gas-exchange space (diameter of alveoli were 3.3 times smaller) and increased the volume density of gas-exchange wall (number of alveoli were 3 times larger) at day 10 after treatment of ATRA compared with that of the rats treated with dexamethasone alone (96). Dexamethasone is a glucocorticosteroid hormone that can inhibit the formation of alveoli, while ATRA is antagonistic with glucocorticosteroid hormone. Thus, we can predict that this antagonistic effect resulted in the protective effects to rats against dexamethasone in this study. More importantly, the rats treated with ATRA alone had a 47% smaller diameter of alveoli and a 50% higher number of alveoli than the rats treated with vehicle, indicating that treatment of ATRA alone led to more adequate gas-exchange functional structures (96). The positive correlation between treatment of ATRA and formation of alveoli suggested a possible regenerative role of ATRA to damaged lung structures, and ATRA activated the endogenous stem/progenitor cells in the lung.
A further study applied ATRA (500 µg/kg/day, i.p., 12 consecutive days) to C57BL/6 mice which had reconstituted bone marrow-derived cells (BMCs) of a GFP-transgenic mice origin (97). ATRA was administered to the mice at 3 weeks after PPE challenge when pulmonary emphysema was generated. ATRA treatment resulted in ameliorated emphysema status shown as smaller mean linear intercept (Lm) of the alveolar spaces compared with that from the mice treated with vehicle. This study confirmed that ATRA can repair structures of alveoli to mice of emphysema. Meanwhile, granulocyte colony stimulating factor (GCSF) was administered either alone or combined with ATRA to the PPE challenged mice being designed as another two groups. GCSF is a glycoprotein that can induce angiogenesis in an ischemia model by stimulating and mobilizing BMCs (98). Immuno-histochemical analysis showed that the GCSF and ATRA combined treatment group had about 10% more GFP+/CD45− BMCs in alveoli of the lung sections compared with that from the GCSF alone treatment group. One cannot be sure whether the regenerated GFP+/CD45− cells were differentiated from BMCs or from fusion of BMCs with the existing parenchyma. But it is clear that ATRA enhanced the effects of GCSF and mobilized more BMCs to the injured alveoli. In summary, treatment of ATRA promoted lung regeneration, increased the number of BMCs in alveoli, and ameliorated emphysema in an elastase-induced COPD model (97). This work supports the notion that ATRA may activate the endogenous stem/progenitor cells in the lung that result in lung structural regeneration.
Clinical results after administering ATRA to activate the endogenous stem/progenitor cells
The discovery that ATRA reversed the functional structures of alveoli in COPD animal models generated interest in studying the clinical effects of ATRA in COPD patients.
A double-blind, placebo controlled, randomized, pilot study applied ATRA (Vesanoid®, Roche) to 20 severe COPD patients (99). This study was not registered at the web of ClinicalTrials.gov. The 20 patients were randomly divided at a ratio of 1:1 to receive either ATRA (25 mg/m2/dose, p.o., twice a day for 4 consecutive days per week) for 12 weeks followed by a matching placebo for 12 weeks, or to receive placebo for 12 weeks followed by ATRA for 12 weeks. Pulmonary function tests, chest CT and quality of life questionnaire were done at baseline, 3 and 6 months after initial treatment with either ATRA or placebo. Results showed that ATRA was well tolerated among all patients, with mild side effects including skin changes (dryness, cracking lips, cheilitis), headache, hyperlipidemia, pruritis, transaminase elevation, muscle and bone pain. Pulmonary function parameters including FEV1 and FEV1/FVC did not have significant changes before and after ATRA treatment. The extent of emphysema observed on chest CT neither changed in response to ATRA over time. The failure of this trial might be due to lower dose, inadequate dosing frequency and dosing method of ATRA. Besides, because ATRA can induce its own oxidative catabolism in vivo, other forms of retinoic acid with similar biological activity but different pharmacokinetics with ATRA may offer better efficacy, and should be considered for future clinical trials.
Palovarotene, a selective retinoic acid receptor gamma agonist, was applied in the “Retinoid treatment of Emphysema in Patients on the α1-antitrypsin International Registry” (REPAIR) trial. It was an investigator-initiated, double-blind, placebo controlled, randomized, multicenter and multinational study that applied palovarotene to severe COPD patients. The two reports of this study did not mention a ClinicalTrials.gov reference number (100,101). However, we found an almost similar study entitled “treatment of emphysema with a gamma-selective retinoid agonist” (TESRA), which was initiated by Roche and was a phase II clinical trial (NCT00413205) at the web of ClinicalTrials.gov. Like ATRA, palovarotene also has been shown to reduce inflammation, promote repair, and improve the function of the emphysematous lung in COPD animal models, probably by activating endogenous stem/progenitor cells in the lung (102). Thus, the “REPAIR” trial can be viewed as another clinical trial on the activation of endogenous stem/progenitor cells in COPD patients in addition to the pilot study described above (99). In the “REPAIR” trial, 110 severe COPD patients were treated with palovarotene (5 mg/day, p.o., for 52 consecutive weeks); another 117 severe COPD patients were treated with placebo in the same period. Palovarotene was well tolerated within the 52-week-treatment-period and in the following 4 weeks. However, treatment of palovarotene did not show significant improvement effects to the lung-density (observed by CT scanning) or function (tested by spirometry) within the treatment-period and in the following 1 year. The authors concluded that 1 year’s treatment of palovarotene might not have been sufficient to achieve clear benefits in severe COPD patients.
There was another clinical trial which applied 13 cis-retinoic acid in COPD patients registered at ClinicalTrials.gov (NCT00000621) and was reported as having been completed, but no results have been presented.
Information on the completed clinical trials that applied small molecules to activate endogenous stem/progenitor cells is given in Table 3.

Full table
Indications from the reported application of ATRA to activate endogenous stem/progenitor cells
Preliminary pre-clinical studies only showed a positive correlation between treatment of ATRA and regeneration of alveoli. Further pre-clinical studies should be done to provide direct evidences to show ATRA can activate endogenous stem/progenitor cells, and to help modify the design of clinical trials in future.
Conclusions
Novel approaches are anticipated to reverse the deterioration of structures and improve the functions of the respiratory system in COPD patients, considering that the currently available drugs mostly focus on relieving the symptoms. Regenerative therapies can improve functionality by repairing or replacing the damaged structures.
Based on data from the large numbers of pre-clinical studies and preliminary clinical trials with MSCs, possible reasons of failures in the clinical trials were discussed above. Major reason of failure could be that the exogenous MSCs had insufficient anti-inflammatory and immunomodulatory effects on the extensively damaged pulmonary structures in moderate-to-severe COPD patients. To enhance the effects, several parameters must be considered including the status of cells (fresh or freezing, from younger or aging donors), dose of cells, dosing methods, frequency of dosing. As to the status of cells, fresh rather than frozen BM-MSCs from younger donors, or AD-MSCs are suggested for future studies. Besides, more efforts should also be given to careful design of new trials including the design of study endpoints, age, status and scale of patient cases, and the inclusion of proper control groups.
For extrinsic cell therapy using exogenous hLSCs and intrinsic cell therapy using molecules that can activate endogenous stem/progenitor cells, further pre-clinical evidence should be gathered before initiating new clinical trials.
In summary, the approaches discussed for regenerative therapies have demonstrated positive effects in COPD animal models and have been safe in clinical trials. However, greater effort must be taken to develop approaches that will lead towards a curing solution to COPD patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: Z Sun and J Wang are current employees and stock option holders of Cellular Biomedicine Group (NASDAQ: CBMG). The other authors have no conflicts of interest to declare.
References
- Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health 2015;5:020415. [Crossref] [PubMed]
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006;27:188-207. [Crossref] [PubMed]
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55. [Crossref] [PubMed]
- Schmidt GA, Girard TD, Kress JP, et al. Official Executive Summary of an American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Am J Respir Crit Care Med 2017;195:115-9. [Crossref] [PubMed]
- Pauwels RA, Buist AS, Ma P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care 2001;46:798-825. [PubMed]
- Moodley YP, Krishnan V, Lalloo UG. Neutrophils in induced sputum arise from central airways. Eur Respir J 2000;15:36-40. [Crossref] [PubMed]
- Finkelstein R, Fraser RS, Ghezzo H, et al. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med 1995;152:1666-72. [Crossref] [PubMed]
- O'Donnell R, Breen D, Wilson S, et al. Inflammatory cells in the airways in COPD. Thorax 2006;61:448-54. [Crossref] [PubMed]
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645-53. [Crossref] [PubMed]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 1996;87:2095-147. [PubMed]
- Lim S, Roche N, Oliver BG, et al. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am J Respir Crit Care Med 2000;162:1355-60. [Crossref] [PubMed]
- Lappalainen U, Whitsett JA, Wert SE, et al. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol 2005;32:311-8. [Crossref] [PubMed]
- Finlay GA, Russell KJ, McMahon KJ, et al. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax 1997;52:502-6. [Crossref] [PubMed]
- Ohnishi K, Takagi M, Kurokawa Y, et al. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest 1998;78:1077-87. [PubMed]
- Taggart C, Cervantes-Laurean D, Kim G, et al. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem 2000;275:27258-65. [PubMed]
- Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J 2008;31:1334-56. [Crossref] [PubMed]
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89. [Crossref] [PubMed]
- Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016;388:963-73. [Crossref] [PubMed]
- Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med 2014;20:822-32. [Crossref] [PubMed]
- Akram KM, Patel N, Spiteri MA, et al. Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches. Int J Mol Sci 2016;17:E128. [Crossref] [PubMed]
- Samadikuchaksaraei A, Bishop AE. Derivation and characterization of alveolar epithelial cells from murine embryonic stem cells in vitro. Methods Mol Biol 2006;330:233-48. [PubMed]
- Rippon HJ, Polak JM, Qin M, et al. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells 2006;24:1389-98. [Crossref] [PubMed]
- Denham M, Cole TJ, Mollard R. Embryonic stem cells form glandular structures and express surfactant protein C following culture with dissociated fetal respiratory tissue. Am J Physiol Lung Cell Mol Physiol 2006;290:L1210-5. [Crossref] [PubMed]
- Wang D, Haviland DL, Burns AR, et al. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 2007;104:4449-54. [Crossref] [PubMed]
- Wang D, Morales JE, Calame DG, et al. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther 2010;18:625-34. [Crossref] [PubMed]
- Zhou Q, Ye X, Sun R, et al. Differentiation of mouse induced pluripotent stem cells into alveolar epithelial cells in vitro for use in vivo. Stem Cells Transl Med 2014;3:675-85. [Crossref] [PubMed]
- Hayes M, Curley G, Ansari B, et al. Clinical review: Stem cell therapies for acute lung injury/acute respiratory distress syndrome - hope or hype? Crit Care 2012;16:205. [Crossref] [PubMed]
- Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res 2008;100:133-58. [Crossref] [PubMed]
- Simonson OE, Domogatskaya A, Volchkov P, et al. The safety of human pluripotent stem cells in clinical treatment. Ann Med 2015;47:370-80. [Crossref] [PubMed]
- Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol 2008;9:725-9. [Crossref] [PubMed]
- Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 1966;16:381-90. [PubMed]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-7. [Crossref] [PubMed]
- Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy 2012;14:516-21. [Crossref] [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Lee M, Jeong SY, Ha J, et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun 2014;446:983-9. [Crossref] [PubMed]
- Schu S, Nosov M, O'Flynn L, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med 2012;16:2094-103. [Crossref] [PubMed]
- Ma N, Gai H, Mei J, et al. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol Int 2011;35:1261-6. [Crossref] [PubMed]
- Liu A, Chen S, Cai S, et al. Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro. PLoS One 2014;9:e90229. [Crossref] [PubMed]
- Liu AR, Liu L, Chen S, et al. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol 2013;228:1270-83. [Crossref] [PubMed]
- Huang K, Kang X, Wang X, et al. Conversion of bone marrow mesenchymal stem cells into type II alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats. Mol Med Rep 2015;11:1685-92. [Crossref] [PubMed]
- Yan C, Qu P, Du H. Myeloid-specific expression of Stat3C results in conversion of bone marrow mesenchymal stem cells into alveolar type II epithelial cells in the lung. Sci China Life Sci 2012;55:576-90. [Crossref] [PubMed]
- Cai SX, Liu AR, Chen S, et al. Activation of Wnt/beta-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res Ther 2015;6:65. [Crossref] [PubMed]
- Loi R, Beckett T, Goncz KK, et al. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med 2006;173:171-9. [Crossref] [PubMed]
- Kim YS, Kim JY, Shin DM, et al. Tracking intravenous adipose-derived mesenchymal stem cells in a model of elastase-induced emphysema. Tuberc Respir Dis (Seoul) 2014;77:116-23. [Crossref] [PubMed]
- Gu W, Song L, Li XM, et al. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci Rep 2015;5:8733. [Crossref] [PubMed]
- Katsha AM, Ohkouchi S, Xin H, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther 2011;19:196-203. [Crossref] [PubMed]
- Johnson M, Koukoulis G, Matsumoto K, et al. Hepatocyte growth factor induces proliferation and morphogenesis in nonparenchymal epithelial liver cells. Hepatology 1993;17:1052-61. [Crossref] [PubMed]
- Panganiban RA, Day RM. Hepatocyte growth factor in lung repair and pulmonary fibrosis. Acta Pharmacol Sin 2011;32:12-20. [Crossref] [PubMed]
- Yanagita K, Matsumoto K, Sekiguchi K, et al. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem 1993;268:21212-7. [PubMed]
- Panos RJ, Patel R, Bak PM. Intratracheal administration of hepatocyte growth factor/scatter factor stimulates rat alveolar type II cell proliferation in vivo. Am J Respir Cell Mol Biol 1996;15:574-81. [Crossref] [PubMed]
- Brechbuhl HM, Li B, Smith RW, et al. Epidermal growth factor receptor activity is necessary for mouse basal cell proliferation. Am J Physiol Lung Cell Mol Physiol 2014;307:L800-10. [Crossref] [PubMed]
- Furuya N, Takenaga M, Ohta Y, et al. Cell therapy with adipose tissue-derived stem/stromal cells for elastase-induced pulmonary emphysema in rats. Regen Med 2012;7:503-12. [Crossref] [PubMed]
- Huh JW, Kim SY, Lee JH, et al. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol 2011;301:L255-66. [Crossref] [PubMed]
- Schweitzer KS, Johnstone BH, Garrison J, et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med 2011;183:215-25. [Crossref] [PubMed]
- Guan XJ, Song L, Han FF, et al. Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem 2013;114:323-35. [Crossref] [PubMed]
- Antunes MA, Abreu SC, Cruz FF, et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res 2014;15:118. [Crossref] [PubMed]
- Hong Y, Kim YS, Hong SH, et al. Therapeutic effects of adipose-derived stem cells pretreated with pioglitazone in an emphysema mouse model. Exp Mol Med 2016;48:e266. [Crossref] [PubMed]
- Ingenito EP, Tsai L, Murthy S, et al. Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Transplant 2012;21:175-89. [Crossref] [PubMed]
- Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42-9. [Crossref] [PubMed]
- Liu X, Fang Q, Kim H. Preclinical Studies of Mesenchymal Stem Cell (MSC) Administration in Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0157099. [Crossref] [PubMed]
- Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther 2012;20:14-20. [Crossref] [PubMed]
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 2012;12:383-96. [Crossref] [PubMed]
- Griffin MD, Ryan AE, Alagesan S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol 2013;91:40-51. [Crossref] [PubMed]
- Hind M, Maden M. Is a regenerative approach viable for the treatment of COPD? Br J Pharmacol 2011;163:106-15. [Crossref] [PubMed]
- Lau AN, Goodwin M, Kim CF, et al. Stem cells and regenerative medicine in lung biology and diseases. Mol Ther 2012;20:1116-30. [Crossref] [PubMed]
- Fang X, Neyrinck AP, Matthay MA, et al. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 2010;285:26211-22. [Crossref] [PubMed]
- Wecht S, Rojas M. Mesenchymal stem cells in the treatment of chronic lung disease. Respirology 2016;21:1366-75. [Crossref] [PubMed]
- Weiss DJ, Casaburi R, Flannery R, et al. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 2013;143:1590-8. [Crossref] [PubMed]
- Ribeiro-Paes JT, Bilaqui A, Greco OT, et al. Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int J Chron Obstruct Pulmon Dis 2011;6:63-71. [Crossref] [PubMed]
- Stessuk T, Ruiz MA, Greco OT, et al. Phase I clinical trial of cell therapy in patients with advanced chronic obstructive pulmonary disease: follow-up of up to 3 years. Rev Bras Hematol Hemoter 2013;35:352-7. [Crossref] [PubMed]
- Cuende N, Rico L, Herrera C. Concise review: bone marrow mononuclear cells for the treatment of ischemic syndromes: medicinal product or cell transplantation? Stem Cells Transl Med 2012;1:403-8. [Crossref] [PubMed]
- Stolk J, Broekman W, Mauad T, et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM 2016;109:331-6. [Crossref] [PubMed]
- Zhen G, Liu H, Gu N, et al. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci 2008;13:3415-22. [Crossref] [PubMed]
- Francois M, Copland IB, Yuan S, et al. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy 2012;14:147-52. [Crossref] [PubMed]
- Broekman W, Roelofs H, Zarcone MC, et al. Functional characterisation of bone marrow-derived mesenchymal stromal cells from COPD patients. ERJ Open Res 2016;2:00045-2015. [Crossref] [PubMed]
- Bonab MM, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cell in vitro. BMC Cell Biol 2006;7:14. [Crossref] [PubMed]
- Beane OS, Fonseca VC, Cooper LL, et al. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One 2014;9:e115963. [Crossref] [PubMed]
- Ahmed AS, Sheng MH, Wasnik S, et al. Effect of aging on stem cells. World J Exp Med 2017;7:1-10. [Crossref] [PubMed]
- Bustos ML, Huleihel L, Kapetanaki MG, et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med 2014;189:787-98. [Crossref] [PubMed]
- Cheng H, Qiu L, Ma J, et al. Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts. Mol Biol Rep 2011;38:5161-8. [Crossref] [PubMed]
- Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A 2005;102:9194-9. [Crossref] [PubMed]
- Chambers SM, Shaw CA, Gatza C, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol 2007;5:e201. [Crossref] [PubMed]
- Beerman I, Bock C, Garrison BS, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell 2013;12:413-25. [Crossref] [PubMed]
- Kajstura J, Rota M, Hall SR, et al. Evidence for human lung stem cells. N Engl J Med 2011;364:1795-806. [Crossref] [PubMed]
- Orlic D, Fischer R, Nishikawa S, et al. Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood 1993;82:762-70. [PubMed]
- Leri A, Kajstura J, Anversa P. Mechanisms of myocardial regeneration. Trends Cardiovasc Med 2011;21:52-8. [Crossref] [PubMed]
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763-76. [Crossref] [PubMed]
- Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A 2007;104:14068-73. [Crossref] [PubMed]
- Bhartiya D. Are Mesenchymal Cells Indeed Pluripotent Stem Cells or Just Stromal Cells? OCT-4 and VSELs Biology Has Led to Better Understanding. Stem Cells Int 2013;2013:547501. [Crossref] [PubMed]
- Rawlins EL, Okubo T, Que J, et al. Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair. Cold Spring Harb Symp Quant Biol 2008;73:291-5. [Crossref] [PubMed]
- Heise RL, Link PA, Farkas L. From Here to There, Progenitor Cells and Stem Cells Are Everywhere in Lung Vascular Remodeling. Front Pediatr 2016;4:80. [Crossref] [PubMed]
- Jarvinen L, Badri L, Wettlaufer S, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 2008;181:4389-96. [Crossref] [PubMed]
- Lama VN, Smith L, Badri L, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 2007;117:989-96. [Crossref] [PubMed]
- Maden M. Retinoids in lung development and regeneration. Curr Top Dev Biol 2004;61:153-89. [Crossref] [PubMed]
- Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol 1996;270:L305-10. [PubMed]
- Ishizawa K, Kubo H, Yamada M, et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249-52. [Crossref] [PubMed]
- Deotare U, Al-Dawsari G, Couban S, et al. G-CSF-primed bone marrow as a source of stem cells for allografting: revisiting the concept. Bone Marrow Transplant 2015;50:1150-6. [Crossref] [PubMed]
- Mao JT, Goldin JG, Dermand J, et al. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med 2002;165:718-23. [Crossref] [PubMed]
- Stolk J, Cooper BG, Stoel B, et al. Retinoid treatment of Emphysema in Patients on the Alpha-1 International Registry. The REPAIR study: study design, methodology and quality control of study assessments. Ther Adv Respir Dis 2010;4:319-32. [Crossref] [PubMed]
- Stolk J, Stockley RA, Stoel BC, et al. Randomised controlled trial for emphysema with a selective agonist of the gamma-type retinoic acid receptor. Eur Respir J 2012;40:306-12. [Crossref] [PubMed]
- Hind M, Stinchcombe S. Palovarotene, a novel retinoic acid receptor gamma agonist for the treatment of emphysema. Curr Opin Investig Drugs 2009;10:1243-50. [PubMed]


