Dosimetric comparison of helical tomotherapy and conventional Linac-based X-knife stereotactic body radiation therapy for primary lung cancer or pulmonary metastases
Introduction
Lung cancer continues to be one of the most prevalent malignancies worldwide and is the leading cause of cancer deaths in both men and women. More than 80% of lung cancers are non-small cell lung cancer (NSCLC) (1). Treating early stage primary NSCLC still requires surgery, while not all of these patients are considered medically operable due to comorbidities, advanced age, or unwillingness to undergo surgery. Besides primary lung cancer, the lung is also one of most common metastatic sites for patients with various malignancies. However, the treatment options are limited for those patients with poor pulmonary function or previously receiving thoracic radiotherapy who are not appropriate for conventional external irradiation (2,3). Furthermore, conventional daily irradiation with a total tumor dose of 66 Gy is also not an adequate option, as it only achieves a 5-year survival rate of 10–30% (4,5).
As a promising new technique, stereotactic body radiation therapy (SBRT) has been introduced as a primary treatment for lung cancer, with promising results in excellent tumor control rates as well as limited toxicities to normal tissue. Since the essential characteristic of SBRT is to deliver a higher biologically effective dose (BED) to tumor target (6-10).
To our knowledge, there are few reports comparing the plans of HT-SBRT and X-SBRT for primary lung cancer or pulmonary metastases. In our study, to address this gap, dosimetric parameters including homogeneity index (HI), conformity index (CI) for planning target volume (PTV), dose and volume parameters of organs at risk (OARs) were compared between the plans of HT-SBRT and X-SBRT for the patients (n=21) with unresectable NSCLC or pulmonary metastases.
Methods
Patients
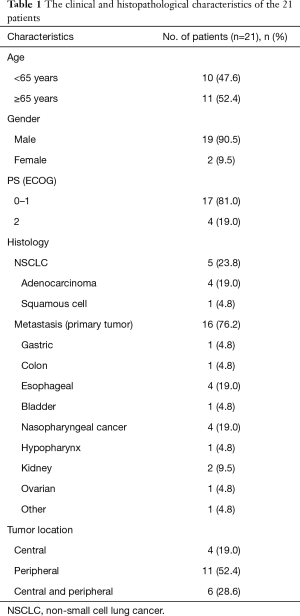
Our study included 21 patients with primary lung cancer and lung oligometastases from various primary sites who underwent SBRT by tomotherapy at our institution between March 2015 and Oct 2016. The enrolled criteria included: 18 years < age < 90 years; maximum diameters of irradiated lesions <5 cm; irradiated lesions ≤2. All patients were newly diagnosed and pathologically confirmed. Five out of the 21 patients were pathologically diagnosed with adenocarcinoma and squamous cell carcinoma. One patient with poor pulmonary function; another patient was 85 years old. Both of them were not suitable for surgery though diagnosed as T1 or T2N0M0 adenocarcinoma. Two patients were stage IV (AJCC 7th edition) adenocarcinoma (intrapulmonary metastasis). The last patient was diagnosed with stage IV squamous cell carcinoma with intrapulmonary recurrence after operation. The other 16 patients were with pulmonary metastases, diagnosed as stage IV. These primary malignances included hypopharynx carcinoma, esophageal cancer, gastric cancer, colon cancer, ovarian cancer, kidney cancer, bladder cancer and nasopharyngeal cancer (NPC). The lung nodules were located in the following areas: 11 patients had a solitary peripheral nodule, 4 had a single central lesion, and 6 had both central and peripheral nodules. All the patients were considered to be inoperable and provided written informed consents. The patients’ characteristics were summarized in Table 1.
Full table
Simulation and delineation of targets and OARs
The computerized tomography (CT) based simulation was used to obtain pictures for the radiation oncologists to map out the tumor and OARs. The scanning range was the total lung, and the layer thickness was 3 mm. The simulation images were transmitted to Pinnacle3 planning system for delineation of the gross tumor volume (GTV), clinical target volume (CTV), PTV and OARs by the same radiation oncologist. Only the primary tumors and metastases in lung were delineated as GTV, and regional (mediastinal) lymph nodes lesions were not included. The OARs included the lung, esophagus, heart and spinal cord.
Treatment planning
The CT images with delineated targets and OARs were transferred to the HI-ART Version 5.0.5.18 treatment planning system and X-knife treatment system (TuoNeng). Then, the physicists designed the HT-SBRT and X-SBRT treatment plans for 21 patients. The isocenter was placed within the tumor volume at the approximate center of mass. The range of collimator of X-knife treatment system was 0.5 to 5 cm. According to the size of the tumor, the range of collimator was selected. X-knife plans were generated using 2–3 coplanar or non-coplanar arcs, according to the location and numbers of the lesions. Such as for a solitary peripheral nodule located in right lower lung, plans angled between 150 and 330 degrees were customized. The computation dose grid size was 3.0 mm. Both groups were prescribed the same dose and fractions. Patients were treated with different fractionation schemes according to the tumor stage (T), tumor location (peripheral vs. central), and primary sites. Tumors were irradiated with 4–10 Gy per fraction in 5–15 fractions. The dose prescriptions were designed to cover at least 96% and 75–85% of the target volume for tomotherapy and X-knife plans, respectively. All plans were assessed and confirmed by senior physicians. The BED for tumor and normal tissues were estimated for each adopted fractionation plan. An α/β ratio equal to 10 Gy was used for primary and secondary tumors, while an α/β equal to 3 Gy was used for late-responding normal tissues. The BED was calculated using the equation nD [1+ D/(α/β)], n is a number of fractions, D is dose per fraction, and α/β was 2 Gy for the spinal cord and 3 Gy for the sparing organs. A median total BED10 was 90.1 Gy (84–100 Gy) at the margin of the PTV.
Dose evaluation
We compared planning parameters by the target dose distribution and OARs. Target coverage was analyzed using the maximum, minimum and mean dose of PTV, HI and CI. Dose distributions of OARs were analyzed using V5, V10, V20, V30 and mean lung dose (MLD), the maximum dose of the spinal cord and mean heart dose.
Statistical analyses
The results of this study were analyzed using the SPSS 16.0. The data were described as the mean ± standard deviation (SD). The paired t-test analysis was performed for comparison of the two groups. Two-sided P≤0.05 was considered statistically significant.
Results
Dose distribution for PTV
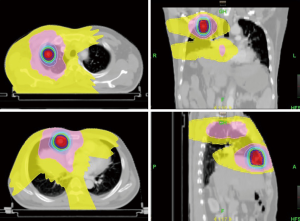
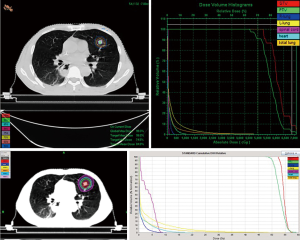
Dose-volume histograms (DVHs) were generated from each planning technique and used to compare PTV coverage and OARs. The dose distribution of PTV by HT and X-knife plan was shown in Table 2 and Figure 1. PTV coverage with the 95% isodose was expected to be better for HT. The values of Dosemax (P<0.001) and Dosemean (P<0.001) were statistically different between the two groups. The difference of Dosemin was not statistically significant (P=0.582).

Full table
Homogeneity and conformity indices of PTV
The homogeneity and the conformal property of the target area of the two plans were shown in Table 3. HI = [D (V2) – D (V98)]/Dpres, where D (V2) and D (V98) equal the dose to 2% and 98% of the target volume respectively, and Dpres represents the prescribed dose. The lower the HI, the more homogenous the dose distribution in PTV. CI values close to 1 indicated a better conformal plan. Regarding the calculated HI and CI, HT was the technique with the most conformed and homogeneous (average values closer to the ideal) dose distribution. Significant differences were observed between the two plans for HI (P=0.003) and CI (P<0.001).

Full table
Dosimetric comparison for OARs
The dose volume parameters of total lung, spinal cord and heart are shown in Table 4. The V5 (P=0.001), V10 (P=0.009), V20 (P=0.001) and MLD (P=0.005) of lung and max spinal cord dose (P=0.01) were significantly lower in the X-SBRT group than the HT-SBRT group. There was no significant difference for the V30 (P=0.075) and mean heart dose (P=0.584).

Full table
Discussion
Standard radiation therapy regimens involving conventional fractionation schemes for early-stage primary lung cancer, advanced localized disease, and lung metastases have delivered only mediocre results so far. So many efforts have been made to achieve high BED while maintaining a sharp dose gradient fall outside the target, preventing dose to critical structures. Therefore, SBRT was developed. SBRT is a non-invasive treatment that can deliver a high dose of radiation in fewer fractions to thoracic or abdominal lesions, which can fully eradicate early-stage primary lung tumors and also can inactivate lung metastases or advanced localized disease. Therefore, it has exclusive advantages in the treatment of tumor peripheral dose change gradient large for pulmonary primary or pulmonary metastases (11-13).
To our knowledge, our study is one of the data to compare SBRT by HT and X-knife for primary lung cancer or pulmonary metastases. This work is an enlightenment to the coming studies and potentially useful for clinicians in making treatment choices for SBRT treatment of lung cancer patients. We found some dosimetric differences. Significant differences between HT and conventional X-knife were observed for HI and CI, and HT can achieve more conformed and homogeneous dose distribution. However, we can accept heterogeneities in SBRT. There was no difference of the V30 of total lung between these two plans. But low dose volumes including V5, V10, V20 and the MLD were significantly lower in the X-SBRT group than in the HT-SBRT group. While some studies also mentioned the volume of total lung with low-dose region (V5–V15) should be carefully regulated for NSCLC patients treated with HT (14). Low-dose irradiation to the lung has been reported to be a risk factor for pulmonary toxicity in lung cancer, especially when combined with chemotherapy (15,16). Our study found the V5 of the total lung in HT-SBRT plans were 26.10%+12.30%, higher than X-knife (Figure 2).
The reason and solution of increased low doses to a large volume of normal lungs have been explained as follows. A general conclusion summarized from previous reports was that advanced multi-beam treatment techniques could improve target homogeneity and reduce high doses, but the distribution of low doses would rise due to increased beam angles (17,18). X-knife use non-coplanar irradiation to protect normal tissue, and then improve the targets dose. However, the range of collimator of X-knife treatment system (TuoNeng) was 0.5 to 5 cm in our institution. So, limited by the diameter of collimator, when PTV diameter is greater than 5 cm, it cannot meet clinical requirements. Tomotherapy was able to achieve better HI and CI; however, this improvement was at the expense of increased volume of the total lung receiving low doses (19-21). In contrast, the conventional irradiation allows radiation to be delivered from only a few directions. The tomotherapy treatment system’s linear accelerator (Linac) is mounted to a CT scanner-like ring gantry, which means it can be delivered continuously, from 360° angles around the patients. So, we use various “block” structures in our plans to reduce the low dose irradiation of lung. By contouring the specific vital structures and setting a “directional block” or “complete block”, beam orientation can be limited to a local region and dose reduction to the normal lung. Directional blocking the majority of the both lung drastically reduces the beam angles used for treatment, therefore, potentially reduces the conformity and normal tissues paring. The block is very helpful to reduce the low dose volume of the total lung. Previous studies also used these options to design other plans (22-24). Moreover, to avoid this inefficiency of beam usage, static ports (TomoDirect® mode) for the treatment of locally advanced lung cancer has been developed, making it possible to reduce the lung volume receiving low dose radiation (25,26). Meanwhile, it is essential to optimize equipment, a newly developed dynamic jaw technology (TomoEDGE) has been introduced. With this technology, radiation doses for the cranio-caudal edges of the target can be lowered by using narrower jaws around the edges (27). Attention to such detail is crucial in HT planning.
As one of the world’s most advanced radiotherapy equipment, some clinical reports about HT-SBRT have demonstrated its feasibility, with a promising outcome and favorable tolerance, especially in the central or multiple targets (28-30). Helical tomotherapy is a novel form of intensity modulated and rotational radiotherapy and image-guided radiation therapy. It uses daily megavoltage computed tomography (MVCT) imaging to guide the treatment daily. During the radiotherapy for thoracic lesions, tumor volume may decrease during the treatment, while the irradiated normal lung tissue volume will increase. So, the application of MVCT can catch the change of tumor volume to achieve adaptive radiotherapy (31). Previous studies also confirmed the safety and effectiveness of tomotherapy for SBRT (32-34).
Conclusions
Helical tomotherapy results in increased low-dose volume of normal tissue and may cause a higher risk of radiation-induced toxicities. X-knife was able to achieve better results than tomotherapy especially in the tumors, maximum diameters of which are less than 5 cm, but had challenges for larger or multiple lesions. It is essential to optimize patient selection in order to reduce the risk of severe radiation pneumonitis in HT-SBRT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This work was approved by the Ethics Committee of the Nanjing Drum Tower Hospital’s (No. 2015-083-02). All the patients were considered to be inoperable and provided written informed consents.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Ball D. Curing Non-Small Cell Lung Cancer with Radiotherapy: No Longer an Oxymoron. Semin Radiat Oncol 2015;25:65-6. [Crossref] [PubMed]
- Xue J, Wang Y. Research progress of precise radiotherapy for lung metastasis. Shandong Medical Journal 2014;54:97-9.
- Sibley GS, Jamieson TA, Marks LB, et al. Radiotherapy alone for medically inoperable Stage I non–small cell lung cancer: The Duke experience. Int J Radiat Oncol Biol Phys 1998;40:149-54. [Crossref] [PubMed]
- Dosoretz DE, Galmarini D, Rubenstein JH, et al. Local control in medically inoperable lung cancer: An analysis of its importance in outcome and factors determining the probability of tumor eradication. Int J Radiat Oncol Biol Phys 1993;27:507-16. [Crossref] [PubMed]
- Xiao J, Xu G, Zhang H, et al. Stereotactic radiotherapy for pulmonary metastatic neoplasm-preliminary experience of 52 patients. Chin J Radiat Oncol 2006:23-7.
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small cell lung cancer: a pooled analysis of two randomized trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004;60:186-96. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Xia T, Li H, Sun Q, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;66:117-25. [Crossref] [PubMed]
- Amini A, Yeh N, Gaspar LE, et al. Stereotactic body radiation therapy (SBRT) for lung cancer patients previously treated with conventional radiotherapy: a review. Radiat Oncol 2014;9:210. [Crossref] [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for T3 and T4N0M0 non-small cell lung cancer. J Radiat Res 2016;57:265-72. [Crossref] [PubMed]
- Westover KD, Iyengar P, Sharma AN, et al. SABR for aggressive local therapy of metastatic cancer: A new paradigm for metastatic non-small cell lung cancer. Lung Cancer 2015;89:87-93. [Crossref] [PubMed]
- Yao B, Wang YD, Liu QZ. Radiation pneumonitis in non-small-cell lung cancer patients treated with helical tomotherapy. Niger J Clin Pract 2016;19:25-9. [Crossref] [PubMed]
- Wang SL, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;64:692-9. [Crossref] [PubMed]
- Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) inpatients with non-small cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys 2006;66:1399-407. [Crossref] [PubMed]
- Landau D, Adams EJ, Webb S, et al. Cardiac avoidance in breast radiotherapy: a comparison of simple shielding techniques with intensity modulated radiotherapy. Radiother Oncol 2001;60:247-55. [Crossref] [PubMed]
- Caudell JJ, De Los Santos JF, Keene KS, et al. A dosimetric comparison of electronic compensation, conventional intensity modulated radiotherapy, and tomotherapy in patients with early-stage carcinoma of the left breast. Int J Radiat Oncol Biol Phys 2007;68:1505-11. [Crossref] [PubMed]
- Schubert LK, Gondi V, Sengbusch E, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy and topotherapy. Radiother Oncol 2011;100:241-6. [Crossref] [PubMed]
- Cattaneo GM, Delloca I, Broggi S, et al. Treatment planning comparison between conformal radiotherapy and helical TomoTherapy in the case of locally advanced - stage NSCLC. Radiother Oncol 2008;88:310-8. [Crossref] [PubMed]
- Meng L, Feng L, Wang Y, et al. Helical tomotherapy for non-small cell lung cancer: A dosimetric study. Journal of Chinese PLA Postgraduate Medical School 2011:52-5.
- Zhang F, Zhang Y, Wang Y. Dosimetric Comparison of Helical Tomotherapy and Conventional Linac-based Intensity Modulated Radiotherapy for Lung Cancer. Chinese Journal of Medical Physics 2012:3596-8.
- Coon AB, Dickler A, Kirk MC, et al. Tomotherapy and multifield intensity-modulated radiotherapy planning reduce cardiac doses in left-sided breast cancer patients with unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys 2010;78:104-10. [Crossref] [PubMed]
- Hodge W, Tome WA, Jaradat HA, et al. Feasibility report of image guided stereotactic body radiotherapy (IG-SBRT) with tomotherapy for early stage medically inoperable lung cancer using extreme hypo fractionation. Acta Oncol 2006;45:890-6. [Crossref] [PubMed]
- Borca VC, Franco P, Catuzzo P, et al. Does TomoDirect 3DCRT represent a suitable option for post-operative whole breast irradiation? A hypothesis-generating pilot study. Radiat Oncol 2012;7:211-21. [Crossref] [PubMed]
- Lee HC, Kim SH, Suh YJ, et al. A prospective cohort study on postoperative radiotherapy with TomoDirect using simultaneous integrated boost technique in early breast cancer. Radiat Oncol 2014;9:244. [Crossref] [PubMed]
- Manabe Y, Shibamoto Y, Sugie C, et al. Helical and Static-port Tomotherapy Using the Newly-developed Dynamic Jaws Technology for Lung Cancer. Technol Cancer Res Treat 2015;14:583-91. [Crossref] [PubMed]
- Baisden JM, Romney DA, Reish AG, et al. Dose as a function of lung volume and planned treatment volume in helical tomotherapy intensity-modulated radiation therapy-based stereotactic body radiation therapy for small lung tumors. Int J Radiat Oncol Biol Phys 2007;68:1229-37. [Crossref] [PubMed]
- Tomita N, Kodaira T, Matsuo M, et al. Helical tomotherapy for solitary lung tumor: feasibility study and dosimetrice valuation of treatment plans. Technol Cancer Res Treat 2010;9:407-15. [Crossref] [PubMed]
- Chi A, Jang SY, Welsh JS, et al. Feasibility of helical tomotherapy in stereotactic body radiation therapy for centrally located dearly stage non-small cell lung cancer or lung metastases. Int J Radiat Oncol Biol Phys 2011;81:856-62. [Crossref] [PubMed]
- Hong TS, Welsh JS, Ritter MA, et al. Megavoltage computed tomography: an emerging tool for image-guided radiotherapy. Am J Clin Oncol 2007;30:617-23. [Crossref] [PubMed]
- Casutt A, Bouchaab H, Beigelman-Aubry C, et al. Stereotactic body radiotherapy with helical TomoTherapy for medically inoperable early stage primary and second-primary non-small-cell lung neoplasm: 1-year outcome and toxicity analysis. Br J Radiol 2015;88:20140687. [Crossref] [PubMed]
- Bral S, Duchateau M, Versmessen H, et al. Toxicity and outcome results of a class solution with moderate lyhypo fractionated radiotherapy in inoperable Stage III non-small cell lung cancer using helical tomotherapy. Int J Radiat Oncol Biol Phys 2010;77:1352-9. [Crossref] [PubMed]
- Sole CV, Lopez Guerra JL, Matute R, et al. Stereotactic ablative radiotherapy delivered by image- guided helical tomotherapy for extracranial oligometastases. Clin Transl Oncol 2013;15:484-91. [Crossref] [PubMed]