The outcomes of anatomical lung resection for nontuberculous mycobacterial lung disease
Introduction
The number of cases of nontuberculous mycobacterial (NTM) lung disease has been increasing in recent years with advances in screening techniques. In 2007, the American Thoracic Society (ATS) revised “An Official Statement: Diagnosis, Treatment and Prevention of Nontuberculous Mycobacterial Diseases” (1). These guidelines state that certain patients might be considered for surgery, including those who show a poor response to drug therapy, disease development, or the presence of significant disease-related complications such as hemoptysis. Although several retrospective studies suggest that surgery can be associated with favorable treatment outcomes (2-7), there are no widely accepted criteria for indicating surgery. Moreover, surgery is associated with a relatively high rate of morbidity. Thus, more data on the treatment outcomes of patients with NTM lung disease who undergo surgical resection will help to determine the optimal role of adjuvant surgery. In addition, only a few studies have aggressively used video-assisted thoracoscopic surgery (VATS) (7,8). We herein report our experience with anatomical surgical resection in the treatment of NTM lung disease and the application of VATS.
Methods
The Institutional Review Board of our hospital approved this retrospective study (approval number: 25-18). The need for subsequent individual consent from patients whose records were evaluated was waived because individuals were not identified in the present study.
The medical and surgical records of 25 patients who underwent anatomical lung resection for NTM lung disease at the National Hospital Organization, Himeji Medical Center from January 2004 and December 2014 were retrospectively reviewed. During this period, non-anatomical resection (i.e., partial resection) for NTM lung disease was performed in 34 patients. Among these 34 cases, there was none in which a definitive diagnosis for NTM was obtained before surgery. We therefore conducted this study focusing only on anatomical lung resections for NTM. The relationship between the two groups was evaluated using the x2 test for categorical variables. The Mann-Whitney U test was used to compare the median value of continuous variables. A two-sided P value <0.05 was considered to be statistically significant.
Preoperative assessment
With the exception of the patients with suspected lung cancer, all of the patients fulfilled the diagnostic criteria and were managed according to the ATS guidelines (1). The patients underwent extensive evaluations, including a sputum analysis and radiological and physiological testing, at their regional hospitals or at our institution. Bronchoscopy or percutaneous needle lung biopsy was performed to confirm the diagnosis and rule out contralateral disease and coexisting malignancy. In most cases, smears and cultures of sputum or bronchial washing samples were examined, and a polymerase chain reaction was performed.
Surgical indications
Surgery for NTM lung disease was performed as part of a multimodal treatment approach. The indications for surgery were generally determined in accordance with “The Guidelines of Surgical Treatment for Pulmonary NTM” (9), which was revised by the Japanese Society for Tuberculosis. As there are no standard definitive criteria for selecting patients for surgery, each case was selected based on a consensus decision by medical and surgical specialists. The standard preoperative work-up included chest radiography, chest computed tomography, a pulmonary functional test, and exercise electrocardiography. Positron emission tomography was added when lung cancer was suspected. We evaluated their lesions, including cavities and bronchiectasis, and their underlying pulmonary condition. Furthermore, we assessed the pulmonary function of the candidates to predict whether they would tolerate resection. Surgical procedures were determined based on a preoperative evaluation and intraoperative findings. The preoperative computed tomography findings revealed the range and distribution of respiratory tract destruction, such as for cavitary and bronchiectatic lesions, and the presence of contralateral lesions. These findings, along with the results of other examinations, helped us decide whether we should perform segmentectomy, lobectomy, or pneumonectomy. The representative computed tomography findings are shown in Figures 1-3. As pneumonectomy is a very invasive procedure, we performed it only in cases in which multiple respiratory tract destruction was found in different lobes or in cases with entire lung destruction (Figure 3). Generally, VATS was performed in cases with no or moderate adhesion, while open thoracotomy was performed in cases in which severe adhesion was predicted (for example, patients with a history of pulmonary resection) or lung destruction that necessitated pneumonectomy; when necessary, VATS was converted to open thoracotomy.

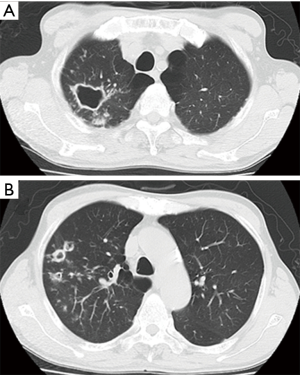
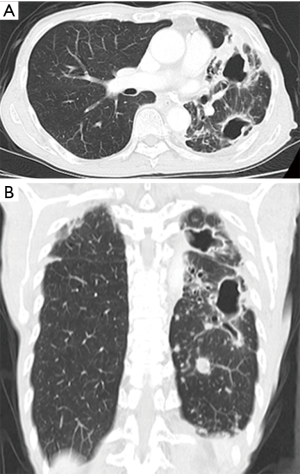
Postoperative management
Routine postoperative care was provided, with an emphasis on pulmonary toilet, pain control, and early ambulation. The postoperative plan was for all patients to be maintained on their multidrug regimen, which was generally the same as the preoperative therapy. The follow-up data were obtained from outpatient or hospital records. Operative mortality was defined as any death that could be directly related to the results of the initial operation, regardless of the postoperative interval. Postoperative complications were defined according to Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; we only considered complications of CTCAE grade ≥2. Air leakage that persisted for more than 7 days was considered to be a postoperative complication.
Results
The clinical summary of 25 patients is shown in Table 1. The patients included 10 men (40.0%) and 15 women (60.0%). The ages of the patients at the time of surgery ranged from 47 to 78 years (average, 63.1 years). The patients’ body mass index values ranged from 14.7 to 29.4 kg/m2 (median, 21.1 kg/m2). Twenty patients were diagnosed with Mycobacterium avium disease (80.0%), and 5 patients were diagnosed with Mycobacterium intracellular disease (20.0%). None of the patients suffered from immunodeficiency disorders such as HIV infection. Preoperative chemotherapy was administered to all 20 cases with a definitive diagnosis (PC group). The patients received the standard combination antibiotic therapy according to the guidelines. The most commonly used regimen consisted of oral macrolide (clarithromycin), ethambutol, and rifampicin. If necessary, streptomycin or kanamycin was included. The duration of preoperative antibiotic therapy typically ranged from 2 months to 2 years, and was occasionally up to 5 years. In five cases without a definitive diagnosis, surgery was performed without preoperative treatment due to the suspicion of lung cancer (PC-free group). Nineteen of the 20 patients in the PC group, had respiratory tract destruction, including cavitary lesions (n=15) or bronchiectatic lesions (n=14), while no patients in the PC-free group had respiratory tract destruction. The indications for lung resection in 20 patients in the PC group included a remaining or worsening lesion despite medical treatment (n=16), massive hemoptysis or bloody sputum (n=5), and prolonged smear positivity (n=1); multiple reasons were allowed.

Full table
The surgical procedures and their outcomes are shown in Table 2. The surgical procedures included pneumonectomy (n=4), lobectomy (n=13), and segmentectomy (n=8). The indications for pneumonectomy were the multiple respiratory tract destruction in different lobes in three patients and entire lung destruction in one patient. Two of the 13 cases of lobectomy were completion lobectomies, and were in the PC-free group, while partial resection was added to 2 of the 8 cases of segmentectomy. On defining complete resection as cases in which an isolated lesion was present or all lesions were included within the resected lung, complete resection was achieved in 10 cases (40.0%) of all 25 cases. Complete resection was achieved in 5 cases (25.0%) in the PC group and in all 5 cases (100.0%) in the PC-free group. In contrast, on defining incomplete resection as cases in which scattered lesions or multiple lesions, including contralateral lesions, remained outside the resected lung, incomplete resection was performed in 15 cases (60.0%) among all cases, accounting for 75.0% in the PC group. VATS procedures were performed in 17 cases (68.0%). In one case, VATS was converted to open thoracotomy due to severe pleural adhesion in a patient undergoing right lower completion lobectomy. Open thoracotomy was performed in 8 cases (32.0%). While open procedures were performed in all cases undergoing completion lobectomy and pneumonectomy, VATS procedures were performed in 6 of 8 cases (75.0%) with segmentectomy and in 10 of 11 cases (90.9%) with simple lobectomy. VATS procedures were used in all five cases in the PC-free group. Pleural adhesion was encountered around the inflammatory lesion in 19 cases (76.0%); no adhesion was encountered in the remaining 6 cases (24.0%). The degree of adhesion in the thoracic cavity was classified into four stages: “none”, defined as no adhesion; “partial”, limited within one lobe; “extensive”, extended over one lobe; and “whole chest wall”. VATS was used in 11 of 14 (78.6%) with no or partial adhesion (Table 2). The median operating time was 127 minutes (range, 65 to 422 minutes); 90 minutes for VATS, and 155 minutes for open thoracotomy. The median intraoperative blood loss was 50 g (range, 10–1,524 g); 15 g with VATS, and 260 g with open thoracotomy. In 3 cases, the blood loss exceeded 500 g (range, 617–1,524 g); 2 of these patients received blood transfusions. The median duration of postoperative drainage was 2 days, and the median postoperative hospital stay was 6 days. Bronchial stump reinforcement with a free pericardial fat pad was performed in 4 cases (right pneumonectomy, n=2; right lower lobectomy, n=2).
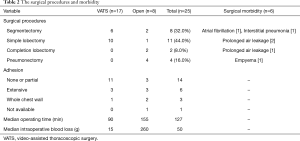
Full table
Surgical morbidities occurred in six cases (24.0%). The most common complication was prolonged air leakage, which occurred in three cases. No cases of bronchopleural fistula were seen in the current study. Although there were no cases of intraoperative mortality, there was one case of hospital mortality. The case involved a 62-year-old man with Mycobacterium avium disease who had been treated with clarithromycin, ethambutol, and rifampicin for 17 months. Despite the provision of treatment, his right-dominant and bilateral lung lesions grew progressively worse and inflammation resulted in lung destruction. He underwent right pneumonectomy as he was completely resistant to chemotherapy and resection was considered to be the only means of halting the progress of the disease. Unfortunately, the patient died 2 months after surgery due to the deterioration of heart and respiratory failure. The right-side heart function on echocardiography of cases in which pneumonectomy was performed is shown in Table 3. In 2 patients, including the deceased patient, the tricuspid regurgitation pressure gradient (TR-PG) was nearly 40 mmHg on postoperative echocardiography, and the acceleration time (AcT)/ejection time (ET) was less than 0.3, which suggested pulmonary hypertension. However, in this deceased patient, the TR-PG was high before surgery, so he may have had pulmonary hypertension before surgery.
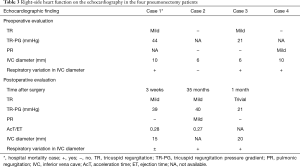
Full table
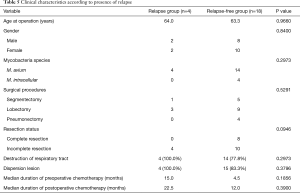
Twenty-two of the 25 patients who underwent anatomical resection were followed in our institution. The median follow-up period was 31.5 months (range, 9 to 97 months). Postoperative chemotherapy was administered in 17 cases (77.3%). All five cases in which postoperative chemotherapy was not administered consisted of patients who underwent complete resection (two in the PC group and three in the PC-free group). Regarding the prognosis of the 22 patients, relapse occurred in 4 patients, and new infection occurred in 1 patient. The disease was controlled in 17 patients (77.3%). One patient with a new infection was a 50-year-old man who had Mycobacterium intracellular lung disease at the time of surgery. He underwent right upper lobectomy due to the presence of a remnant cavitary lesion after three months’ of treatment with clarithromycin, ethambutol, and rifampicin. The same antibiotic regimen was maintained after surgical resection and was continued for 12 months. Two years later, a nodular bronchiectatic lesion was gradually recognized in his left upper lobe, and a sputum culture was positive for Mycobacterium avium. We therefore considered this to be a new infection. The four cases that relapsed are shown in Table 4. In all of these cases, the size of the remaining lesion increased after incomplete resection. The median duration of preoperative chemotherapy was 15 months. Postoperative chemotherapy was administered to all four patients. In Case 1, the postoperative chemotherapy was stopped at just 3 months after surgery due to pruritus. In Case 2, a contralateral lesion that had initially improved with postoperative chemotherapy deteriorated during chemotherapy. The median relapse-free interval was 29.5 months (range, 24 to 55 months). Table 5 depicts the distribution of the clinical characteristics according to the presence of relapse. Relapse was not associated with the surgical procedure or the presence of destruction of the respiratory tract. However, none of the patients with relapse had undergone complete resection, although there was no statistical significance in this finding. Although there were no statistically significant differences in any factors, the recurrence group tended to have a longer pre- and post-operative chemotherapy duration than the non-recurrence group. Even when examining the same factors according to the presence of relapse in only the PC group, similar results were obtained.

Full table
Full table
Discussion
There are two aims of surgery for NTM lung disease. The first aim is to make multidrug therapy more effective by removing the areas of the lung that are most affected and that harbor the largest amounts of mycobacteria; this reduces the load of mycobacteria on the patient’s body. The other aim is to remove areas of the respiratory tract that show destructive changes such as cavitation or bronchiectasis, where recurrence or relapse often occurs. According to the Japanese surgical treatment guidelines (9), the indications for surgical resection include ineffective chemotherapy, relapse, deterioration with massive mycobacterial expectoration, and destructive changes such as cavitation or bronchiectasis. In our institution, the indications for resection were determined based on these guidelines. Under these guidelines, if the main cavity or mass lesion that is the main source of bacterial discharge can be removed, incomplete resection is also included in the surgical indication. Although relapse occurred in four cases in which incomplete resection was performed, 77.3% of the surgical cases obtained stable disease or an improvement after surgery. Because we performed surgery for the cases in which improvement could not be expected and in which stable disease was not obtained by medical treatment alone, it is thought that surgical treatment—including incomplete resection—is effective. Regarding the single in-hospital mortality, the patient may have had pulmonary hypertension before surgery. Preoperative pulmonary hypertension and subsequent pneumonectomy might have influenced his postoperative heart failure. Patients requiring pneumonectomy generally have a poor general condition due to progression of their disease and long-term treatment. We must carefully consider the indication of surgery, especially for pneumonectomy, in the future.
The optimal duration for which chemotherapy should be administered before assessing a patient for surgery remains unclear (2). In a study by Watanabe et al., the patients received a clarithromycin-containing regimen for more than 6 months in order to treat Mycobacterium avium-intracellulare complex pulmonary disease with cavitary or bronchiectatic lesions should be viewed as candidates for surgery (10). Shiraishi et al. recommended that patients with localized lesions be considered for pulmonary resection as early as possible (5). In the present study, the duration varied from months to years, and no definitive results were obtained; however, in all four cases that required pneumonectomy, preoperative chemotherapy was administered for more than one year. Considering that there was only one case of hospital mortality, in a patient who required pneumonectomy, and the fact that relapse occurred in four cases in patients who underwent incomplete lobectomy or segmentectomy, it might be necessary to perform surgery before the disease becomes too advanced. In this study, we detected no statistically significant differences in features on radiological findings between the relapse and relapse-free groups due to the small number of samples. However, in the relapse group, the duration of preoperative chemotherapy (median 15 months) tended to be longer than that of the relapse-free group. These results also suggest, as mentioned above, that surgery should be considered at an early stage before the disease progresses and becomes difficult to resect. The duration of postoperative chemotherapy also tended to be longer in the relapse group than in the relapse-free group. The significance of these findings should be discussed in the future. The relapse group may have been infected with bacterial species resistant to chemotherapy, which might have lengthened the treatment period and caused relapse. Furthermore, five cases with presumed lung cancer before surgery had an isolated lesion, and complete resection was obtained. As there were no instances of relapse without pre- and postoperative chemotherapy in these five cases, it is obvious that complete resection is desirable. Not needing chemotherapy after surgery for cases with isolated lesions able to be resected completely would therefore be very useful for such patients.
Despite the favorable treatment outcomes, the rate of postoperative complications was relatively high in our study. This rate is similar to those of previous studies (3,6,7,11,12) that reported a high incidence of postoperative morbidity. In previous studies (2-4,6-8,10-12), the performance of pneumonectomy was correlated with the frequency of complications. In our study, pneumonectomy was performed in four cases (16.0%). Mitchell et al. reported that major and minor morbidity rates were 11.7% and 6.8%, respectively. Their most common pattern of focal parenchymal lung disease was bronchiectasis, which was seen in 55% of patients; cavitary lesions, including lung destruction, were only seen in 29% of cases (3). In our study, cavitary lesions were seen in more cases. The high ratio of advanced disease, such as cavity formation and the need to perform pneumonectomy might have led to the relatively high rate of postoperative morbidity in our study.
VATS has been more widely used in patients with NTM lung disease, especially those with nodular bronchiectatic form, in recent years (8). In the present study, 68.0% of the cases in which resection was performed were performed by VATS. In particular, 75.0% of segmentectomies and up to 90.9% of simple lobectomies were performed with VATS. This was associated with a shorter operative time and lower intraoperative blood loss in comparison to open thoracotomy. Only one case (5.9%) was converted from VATS to open thoracotomy. The application of VATS to the surgical treatment of infectious disease was quite difficult due to severe adhesion and lymphadenopathy. However, in cases requiring simple anatomical resection without dense pleural adhesion, surgery with VATS is believed to be possible. The use of VATS was associated with less morbidity, a better functional status, and an improved ability to deliver subsequent medical therapy (13,14). We are of the opinion that minimally invasive approaches (including VATS) are useful for the surgical treatment of NTM lung disease, and we believe that the indications for VATS for NTM will be expanded in the future.
Several limitations are associated with the present study. First, this retrospective study was conducted at a single institution, and the surgical patients were specifically selected. Second, there was no surveillance protocol, so the follow-up approach may have differed among doctors. Furthermore, the present study analyzed relatively few cases. In the future, we need to accumulate more cases and long-term follow-up data, leading to more objective assessment.
In summary, although there was 1 case of hospital mortality, anatomical lung resection for pulmonary NTM lung disease was feasible, with acceptable mortality rates, and favorable treatment outcomes. Surgical treatment, including VATS, might be indicated for pulmonary NTM after careful consideration.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board of National Hospital Organization Himeji Medical Center approved this retrospective study (approval number: 25-18). The need for subsequent individual consent from patients whose records were evaluated was waived because individuals were not identified in the present study.
References
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. [Crossref] [PubMed]
- Shiraishi Y, Katsuragi N, Kita H, et al. Adjuvant surgical treatment of nontuberculous mycobacterial lung disease. Ann Thorac Surg 2013;96:287-91. [Crossref] [PubMed]
- Mitchell JD, Bishop A, Cafaro A, et al. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg 2008;85:1887-92; discussion 1892-3.
- Shiraishi Y, Nakajima Y, Katsuragi N, et al. Pneumonectomy for nontuberculous mycobacterial infections. Ann Thorac Surg 2004;78:399-403. [Crossref] [PubMed]
- Shiraishi Y, Fukushima K, Komatsu H, et al. Early pulmonary resection for localized Mycobacterium avium complex disease. Ann Thorac Surg 1998;66:183-6. [Crossref] [PubMed]
- Nelson KG, Griffith DE, Brown BA, et al. Results of operation in Mycobacterium avium-intracellulare lung disease. Ann Thorac Surg 1998;66:325-30. [Crossref] [PubMed]
- Kang HK, Park HY, Kim D, et al. Treatment outcomes of adjuvant resectional surgery for nontuberculous mycobacterial lung disease. BMC Infect Dis 2015;15:76. [Crossref] [PubMed]
- Mitchell JD, Yu JA, Bishop A, et al. Thoracoscopic lobectomy and segmentectomy for infectious lung disease. Ann Thorac Surg 2012;93:1033-9; discussion 1039-40. [Crossref] [PubMed]
- Nontuberculous Mycobacteriosis Control Committee of the Japanese Society for Tuberculosis. International Exchanging Committee of the Japanese Society for Tuberculosis. Guidelines for surgical therapy for pulmonary nontuberculous mycobacterial diseases. Kekkaku 2011;86:41-2. [PubMed]
- Watanabe M, Hasegawa N, Ishizaka A, et al. Early pulmonary resection for Mycobacterium avium complex lung disease treated with macrolides and quinolones. Ann Thorac Surg 2006;81:2026-30. [Crossref] [PubMed]
- Pomerantz M, Madsen L, Goble M, et al. Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg 1991;52:1108-11; discussion 1112. [Crossref] [PubMed]
- Koh WJ, Kim YH, Kwon OJ, et al. Surgical treatment of pulmonary diseases due to nontuberculous mycobacteria. J Korean Med Sci 2008;23:397-401. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Lee JG, Cho BC, Bae MK, et al. Thoracoscopic lobectomy is associated with superior compliance with adjuvant chemotherapy in lung cancer. Ann Thorac Surg 2011;91:344-8. [Crossref] [PubMed]


