Selective serotonin reuptake inhibitor use and outcomes following cardiac surgery—a systematic review
Introduction
The prevalence of depression in the general population of the UK has risen in the past two decades and continues to rise at a significant rate (1). Consequently, the prescription of antidepressant medication by general practitioners has almost doubled in this time period. Importantly, patients with coronary artery disease have a higher prevalence of depression than the general population, estimated around 20–40%, with the highest rates of depression found in those awaiting coronary artery bypass graft surgery (CABG) (2,3). The knowledge of the need for an imminent operation contributes to the physical and psychological stress associated with depression (4-6). Depression has been shown to be associated with a higher risk of morbidity and mortality following acute coronary syndromes (7) and subsequent cardiac surgery (8). Additionally, depressed patients have been found to be more likely to present with complications following cardiac surgery including recurrent angina, heart failure and repeat revascularization (9). The current first line treatment for the management of depressive illness and panic disorders are selective serotonin re-uptake inhibitors (SSRIs), having demonstrated a more acceptable side-effect profile and comparable efficacy to the traditional tricyclic antidepressants (TCAs). The primary site of action of SSRIs is the blockade of the serotonin transporter in cortical neurons (10). Additionally, they influence platelet serotonin transport thereby rendering platelets unable to synthesise intrinsic serotonin. This leaves platelets dependent on the uptake of serotonin from their environment therefore amplifying the importance of the serotonin transporter in serotonin-mediated platelet activation (11,12). Peri-operative blood loss and the need for blood transfusion in patients undergoing cardiac surgery are associated with an increased risk of post-operative morbidity and mortality (13,14). Despite the prevalence of depression and SSRI use in patients undergoing cardiac surgery, the short- and long-term safety and outcome profile of this class of medication has not been rigorously analysed in this patient cohort.
The aims of this study are to address the following questions: (I) is the use of SSRIs associated with a higher rate of mortality and major adverse events following cardiac surgery? (II) Is the use of SSRIs associated with increased bleeding events following cardiac surgery?
Methods
Search
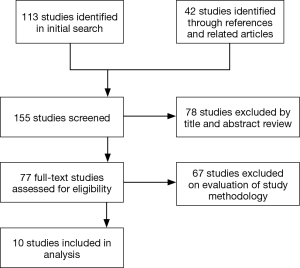
A literature search was performed using PubMed, Ovid, EmBase and Google Scholar up to January 2017 using the MESH headings (SSRIs OR SSRI) AND ((cardiac OR thoracic) AND (surgery)). Cochrane Central Register of Controlled Trials (CENTRAL/CCTR) and ClinicalTrials.gov were also searched. Related articles and references were screened for suitable articles. Studies in English that analysed outcomes in patients taking SSRIs and undergoing cardiac surgery were included. 113 studies were identified in the initial search, with a further 42 studies identified through references and related articles. Of these studies, 78 were excluded by title and abstract review. Of the remaining 77 studies, 67 were excluded following full-text review as they had not directly compared the use of SSRIs with no SSRI use in patients undergoing cardiac surgery (Figure 1). Data extraction was performed independently by two authors (AS and ME).
Outcomes of interest
Outcomes of interest were 30-day mortality, major adverse events, bleeding events and re-operation for bleeding.
Results
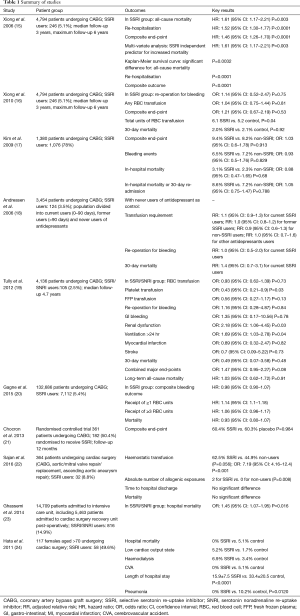
Ten studies were identified by systematic search to fulfil the inclusion criteria (Table 1) (15-24).
Full table
Mortality
All ten studies (15-24) reported the outcomes of either 30-day hospital or all-cause mortality, or long-term survival. Two studies (15,23) reported higher mortality in the SSRI group. Xiong et al. (15) reported a significantly higher all-cause mortality [hazard ratio (HR): 1.61, 95% confidence interval (CI): 1.17–2.21, P=0.003] in the SSRI group, with Kaplan-Meier survival curves demonstrating a significantly increased all-cause mortality (P=0.0032) in the SSRI group. Ghassemi et al. (23) reported significantly higher hospital mortality [odds ratio (OR): 1.45, 95% CI: 1.07–1.95, P=0.016] in the SSRI group. Seven other studies (16-20,22,24) reported no significant difference in 30-day or hospital mortality, and of these studies 3 studies (19,20,22) and 1 additional study (21) reported no significant difference in all-cause mortality between SSRI and non-SSRI groups.
Bleeding events
Seven studies (16-22) reported bleeding events, including re-operation for bleeding or tamponade, requirement for red blood cell (RBC) transfusion, requirement for haemostatic agent [platelets, fresh frozen plasma (FFP), cryoprecipitate] transfusion and GI haemorrhage. Two studies (16,22) reported higher bleeding events in the SSRI group. Xiong et al. (16) reported the total units of post-operative RBC transfused to be higher in the SSRI group (6.1 vs. 5.2, P=0.04). Sajan et al. (22) reported a significantly higher risk associated with SSRI use and the receipt of haemostatic transfusion (OR: 7.19, 95% CI: 4.16–12.4, P<0.001). The five other studies (17-21) reported no significant difference in any of the above bleeding events between the SSRI and non-SSRI groups.
Other morbidity
Five studies (15,17,19,21,24) reported other morbidity events including MI, stroke, re-operation for any cause, requirement for new dialysis, ventilation >24 hours, sternal wound infection and re-hospitalisation. One study (19) reported a significantly higher rate of dialysis and ventilation >24 hours (OR: 2.18, 95% CI: 1.06–4.45, P=0.03) and (OR: 1.69, 95% CI: 1.03–2.78, P=0.4) respectively in the SSRI group. Another study (15) reported a significantly higher rate of re-hospitalisation (HR: 1.52, 95% CI: 1.30–1.77, P<0.0001) in the SSRI group. The other three studies (Kim, Chocron, Hata) reported no significant increase in the rate of any morbidity in the SSRI group. Hata et al. (24) reported significantly lower hospital lengths of stay (15.9±7.5 vs. 33.4±20.5, P<0.0001) and rates of pneumonia (0% vs. 10.2%, P=0.0120) in the SSRI group.
Studies
Xiong et al. (15) performed a retrospective observational analysis of 4,794 patients undergoing CABG. Pre-operative SSRI use was reported in 246 (5.1%) of the study population. Primary outcomes of interest were event-free survival from all-cause mortality, re-hospitalisation, and the composite outcome of all-cause mortality or re-hospitalisation. All-cause mortality (HR: 1.61, 95% CI: 1.17–2.21, P=0.003], re-hospitalisation (HR: 1.52, 95% CI: 1.30–1.77, P<0.0001) and the composite endpoint (HR: 1.46, 95% CI: 1.26–1.70, P<0.0001) were increased significantly in the SSRI group compared to those not taking SSRIs preoperatively.
Xiong et al. (16) performed a second analysis of the same study population as the previous study. This time the primary outcomes of interest were re-operation for bleeding complications. Secondary outcomes of interest were 30-day mortality; the need for post-operative RBC transfusion; and the composite end-point of re-operation for bleeding complications, post-operative haematocrit drop of >15%, or any post-operative RBC transfusion. The pre-operative use of SSRIs before CABG was not significantly associated with increased odds of re-operation for bleeding complications (OR: 1.14, 95% CI: 0.52–2.47, P=0.75), for any RBC transfusion (OR: 1.04, 95% CI: 0.75–1.44, P=0.81), and for the composite end-point (OR: 1.21, 95% CI: 0.67–2.19, P=0.53). The adjusted total units of post-operative RBC transfused was 6.1 in the SSRI group and 5.2 in the control group (P=0.04).
Kim et al. (17) conducted a retrospective study of 1,380 patients receiving antidepressant medication prior to CABG, 1,076 (78%) of whom received SSRIs preoperatively. Primary outcomes of interest were the composite outcome of in-hospital mortality or any bleeding events (post-procedural haemorrhage or haematoma, GI hemorrhage, and re-opening of surgical site). Secondary outcomes of interest included each component of the primary composite outcome, as well as 30-day readmission. None of the primary or secondary outcomes of interest were significantly different between the two groups. The composite outcome occurred in 9.4% in the SSRI group versus 8.2% in the non-SSRI group (OR: 1.03, 95% CI: 0.60–1.78, P=0.913). Bleeding events occurred in 6.5% in the SSRI group versus in 7.2% in the non-SSRI group (OR: 0.93, 95% CI: 0.50–1.76, P=0.829). In-hospital mortality occurred in 3.1% in the SSRI group versus in 2.3% in the non-SSRI group (OR: 0.88, 95% CI: 0.47–1.65, P=0.68). In-hospital mortality or 30-day re-admission occurred in 8.6% in the SSRI group versus in 7.2% in the non-SSRI group (OR: 1.05, 95% CI: 0.75–1.47, P=0.788). These results were not significantly different when comparisons were made between the rate of primary and secondary outcomes of interest in the following groups of patients: (I) those in whom antidepressant medication was discontinued on the day of or 1 day before surgery and was not resumed during the hospitalisation (OR: 1.11, 95% CI: 0.46–2.68, P=0.810); (II) those in whom antidepressant medication was continued or resumed after surgery (OR: 1.14, 95% CI: 0.68–1.93, P=0.618); and (III) those who received antiplatelet and anticoagulation therapy for acute coronary syndromes (OR: 1.03, 95% CI: 0.40–2.61, P=0.958).
Andreasen et al. (18) conducted a study of 3,454 patients undergoing CABG of whom 124 (3.5%) were current users of SSRIs. The antidepressants were categorised into three groups: SSRIs, non-SSRIs (SRIs, antidepressants with an inhibitory effect on both serotonin and norepinephrine re-uptake), and other antidepressants. Outcomes of interest were transfusion requirements [including RBCs, FFP or platelets] re-operation and mortality. Considering transfusion requirements, using the never users of any type of antidepressant as the reference group, the adjusted relative risk (RR) for transfusion among current SSRI users was 1.1 (95% CI: 0.9–1.3). Similarly, no increased requirement for transfusion was found among former users of SSRI (RR: 1.0, 95% CI: 0.8–1.2), current users of non-selective SRIs (RR: 0.9, 95% CI: 0.6–1.3), or current users of other antidepressants (RR: 1.1, 95% CI: 0.7–1.6). Similar outcomes were observed when the analyses were focused on specific blood components (e.g., RBC, FFP, or platelets). Re-operation as a result of bleeding was not associated with the use of antidepressants, including SSRIs, with the adjusted RR for re-operation 1.0 (95% CI: 0.5–2.0) among current SSRI users. The overall 30-day mortality was 4.2% and no clear association between the use of antidepressants and 30-day mortality was demonstrated. The adjusted RR for 30-day mortality was 1.4 (95% CI: 0.7–3.1) amongst current SSRI users.
Tully et al. (19) conducted a retrospective study of 4,136 patients undergoing CABG, of whom 105 (2.5%) were SSRI or serotonin noradrenaline re-uptake inhibitor (SNRI) users. Outcomes of interest were bleeding events (transfusion of RBC, FFP or platelets; re-operation for bleeding; GI bleeding), new renal failure, stroke, ventilation >24 hours, deep sternal wound infection, re-operation (for any cause), myocardial infarction (MI) and mortality. There was no association between SSRI/SNRI use and bleeding events apart from the need for platelet transfusion, which was significantly lower for the SSRI/SNRI users (RBC transfusion: OR: 0.93, 95% CI: 0.62–1.39, P=0.73), (platelet transfusion: OR: 0.43, 95% CI: 0.21–0.9, P=0.03), (FFP transfusion: OR: 0.56, 95% CI: 0.27–1.17, P=0.13), (re-operation for bleeding: OR: 1.16, 95% CI: 0.28–4.87, P=0.84), (GI bleeding: OR: 1.35, 95% CI: 0.17–10.56, P=0.78). There was a significantly higher rate of renal dysfunction and need for dialysis and ventilation >24 hours associated with the use of SSRI/SNRI (OR: 2.18, 95% CI: 1.06–4.45, P=0.03) and (OR: 1.69, 95% CI: 1.03–2.78, P=0.04) respectively. There was no association between SSRI/SNRI use and all-cause mortality on long-term follow-up (HR: 1.03, 95% CI: 0.62–1.72, P=0.91).
Gagne et al. (20) conducted a retrospective study of 132,686 patients undergoing CABG, comprising of 7,112 (5%) patients receiving SSRIs prior to surgery, 1,905 (1%) patients receiving other antidepressants prior to surgery, and 123,669 (94%) not receiving antidepressant treatment. Primary outcomes of interest were a composite bleeding outcome including RBC transfusion of >3 units, any platelet transfusion, any FFP transfusion, any cryoprecipitate transfusion, and GI bleeding. Neither the SSRI group (HR: 0.98, 95% CI: 0.80–1.07) nor the other antidepressant group (HR: 1.11, 95% CI: 0.96–1.28) had an increased major bleeding risk in the primary multivariable adjusted model, as compared to the no exposure group. Neither SSRIs nor other antidepressants were associated with significantly increased rates of any of the individual outcomes comprising the composite bleeding outcome. Both the SSRI group (HR: 1.14, 95% CI: 1.10–1.18) and the other antidepressant group (HR: 1.11, 95% CI: 1.03–1.19) were associated with a slight increase in the receipt of ≥1 RBC units, but neither were associated with significant increases in receipt of ≥3 RBC units (HR: 1.06, 95% CI: 0.96–1.17 and HR: 1.09, 95% CI: 0.91–1.31 respectively). The risk of mortality was similar for SSRI users (HR: 0.93, 95% CI: 0.80–1.07) and other antidepressant users (HR: 0.84, 95% CI: 0.62–1.14).
Chocron et al. (21) conducted a single-centre randomised controlled trial of 361 patients undergoing CABG, with 182 patient randomised to receive an SSRI and 179 patients randomised to receive a placebo from 2–3 weeks prior to surgery to 6 months after surgery. Follow-up was 12 months. The primary composite endpoint was the occurrence of mortality or morbidity events in the 12-month post-operative period. Morbidity events were defined as 1 or more of the following: (I) cardiac: peri-operative MI or low cardiac output syndrome; (II) pulmonary: mechanical ventilation support >24 hours, or need for re-intubation; (III) neurologic: focal brain injury with permanent or transient deficit, or agitation or confusion >24 hours; (IV) renal: need for dialysis when previously not required, or maximum creatinine serum level more than twice preoperative creatinine serum level; (V) acquired atrial fibrillation: paroxysmal or persistent; (VI) infectious: pneumonia, sepsis with positive culture, sternal wound infection requiring intravenous antibiotics, surgical debridement, or both; (VII) any surgery or invasive procedure necessary to treat a postoperative adverse event associated with the initial cardiac surgery; (VIII) MI; (IX) congestive heart failure; (X) re-hospitalisation for cardiac-related cause; and (XI) re-hospitalisation for non-cardiac related cause. By the end of the follow-up period there were no differences in the composite endpoint between the two groups [110 of 182 (60%) vs. 108 of 179 (60%) respectively, P=0.984] nor were there any differences in the individual events that defined the composite endpoint between the two groups.
Sajan et al. (22) conducted a prospective study of 364 patients undergoing cardiac surgery (CABG, aortic/mitral valve repair/replacement, ascending aortic aneurysm repair), of whom 32 patients were taking SSRIs. The primary outcome of interest was the transfusion of any haemostatic allogenic blood product (FFP, platelets, cryoprecipitate) intra-operatively and post-operatively through day 2. Secondary outcomes of interest were a composite of all allogenic exposures (FFP, platelets, cryoprecipitate and RBC), time to hospital discharge and mortality at 32 days. In the unadjusted analysis the proportion receiving haemostatic transfusions was 62.5% for the SSRI group and 44.9% for non-users (P=0.056). In terms of the absolute number of allogenic exposures, the number of exposures (median, 25–75%) was significantly greater among the SSRI group than the non-users [2 (0–6) vs. 0 (0–2), for SSRI and no SSRI respectively, P=0.008]. With multivariate adjustment, there was a significantly higher risk associated with SSRI use and the receipt of haemostatic transfusion (OR: 7.19, 95% CI: 4.16–12.4, P<0.001). There were no significant differences observed in the time to hospital discharge and mortality between SSRI users and non-users.
Ghassemi et al. (23) conducted a retrospective study of 14,709 patients admitted to an intensive care unit, of whom 5,463 patients were admitted to the cardiac surgery recovery unit post-operatively (including 816 SSRI/SNRI users and 4,647 non-users). The outcome of interest was in-hospital mortality. There was a significantly higher rate of mortality observed in those admitted to the cardiac surgery recovery unit amongst the SSRI/SNRI users (OR: 1.45, 95% CI: 1.07–1.95, P=0.016).
Hata et al. (24) conducted a retrospective study of 117 female patients over the age of 70 undergoing cardiac surgery, of whom 58 patients were given prophylactic SSRI post-operatively and 59 patients were controls. Outcomes of interest were the duration of hospital stay; the incidences of cerebrovascular complications, pneumonia and hospital mortality between the two groups. There were no significant differences in hospital mortality (0% vs. 5.1%), low cardiac output state (5.2% vs. 1.7%), haemodialysis (6.9% vs. 3.4%), cerebrovascular accident (CVA) (0% vs. 5.1%) between the SSRI group and the control group respectively. There were significantly lower lengths of stay (15.9±7.5 vs. 33.4±20.5, P<0.0001) and pneumonia (0% vs. 10.2%, P=0.0120) in the SSRI group.
Discussion
We have conducted a review of ten studies comprising 162,001 patients (SSRI use in 9,751 patients) undergoing cardiac surgery, in whom the use of SSRIs pre- or post-operatively were analysed with respect to the outcomes of interest of mortality, bleeding events and other significant morbidity. All studies reported mortality and 8 of these studies demonstrated no significant difference in 30-day, hospital or long-tern mortality between the SSRI and non-SSRI groups. Depression prior to cardiac surgery has been repeatedly associated with increased mortality and cardiac events (3-8). Long-term survival analyses in these studies have demonstrated that patients with moderate or severe depression before cardiac surgery have a 2.4-fold increase in mortality compared to a non-depressed population (3). It has also been shown that patients with depression that persisted from baseline to 6 months post-operatively had almost double the mortality rate than those not suffering from depression (3,4). Considering these associations, there are strong indications that the use of antidepressants and specifically SSRIs can alleviate not only the depressive disorder but also improve the cardiovascular prognosis of patients with depression (25). Additionally, SSRIs do not exhibit the adverse cardiovascular effects of other antidepressants, particularly the pro-arrhythmic effects of the tricyclic antidepressant. Seven studies reported bleeding events and 5 of these studies reported no significant difference in any of the bleeding events between the SSRI and non-SSRI groups. SSRIs have antiplatelet activity and reduce the release of platelet factor 4, B-thromboglobulin and P-selectin (26-28). Platelet dysfunction is caused by the inhibition of granule release and subsequent adenosine diphosphate-induced platelet aggregation. Clinical studies have previously demonstrated increased incidence of GI bleeding (29,30) and increased transfusion requirements in patients undergoing orthopaedic surgery (31) associated with SSRI use. It must however be considered that patients undergoing cardiac surgery are frequently receiving some form of anti-platelet therapy and may also exhibit platelet dysfunction post-operatively secondary to the effects of cardiopulmonary bypass. Consequently, it is possible that the additional anti-platelet effect of SSRIs may not add significantly to the short-term risk of bleeding in these patients. Five studies reported other significant morbidities and 3 of these studies reported no difference in significant morbidity between the SSRI and non-SSRI groups. It is postulated that the serotonin transporter affinity and attenuation of platelet function associated with SSRI use can provide cardiac benefits such as reduced incidences of myocardial ischaemia or infarction as well as providing protection against other thromboembolic events, potentially impacting long-term survival. One study reported a significantly higher rate of dialysis and ventilation >24 hours in the SSRI group. The authors concluded that these findings provided the first documented associations between SSRI/SNRI use and increased requirement for renal dialysis and extended ventilation following CABG surgery, and consequently the results must be analysed with caution as they may reflect the underlying effects or associated factors of depression and anxiety rather than SSRI/SNRI use.
Our study reviews the evidence and demonstrates the safety of the use of SSRIs for the treatment of depressive disorders in patients undergoing cardiac surgery. Whilst there are some reports of increased mortality and bleeding events with the use of SSRIs, the significant body of evidence does not support these findings. The limitations of our review include the retrospective nature of the studies analysed, incomplete or unavailable data, and the risk of reporting bias. The need for larger randomized studies to assess the relationship between SSRI use and outcomes following cardiac surgery does remain.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- King M, Nazareth I, Levy G, et al. Prevalence of common mental disorders in general practice attendees across Europe. Br J Psychiatry 2008;192:362-7. [Crossref] [PubMed]
- Celano CM, Huffman JC. Depression and cardiac disease: a review. Cardiol Rev 2011;19:130-42. [Crossref] [PubMed]
- Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003;362:604-9. [Crossref] [PubMed]
- Burker EJ, Blumenthal JA, Feldman M, et al. Depression in male and female patients undergoing cardiac surgery. Br J Clin Psychol 1995;34:119-28. [Crossref] [PubMed]
- Stoll C, Schelling G, Goetz AE, et al. Health-related quality of life and post-traumatic stress disorder in patients after cardiac surgery and intensive care treatment. J Thorac Cardiovasc Surg 2000;120:505-12. [Crossref] [PubMed]
- Pirraglia PA, Peterson JC, Williams-Russo P, et al. Depressive symptomatology in coronary artery bypass graft surgery patients. Int J Geriatr Psychiatry 1999;14:668-80. [Crossref] [PubMed]
- Meijer A, Conradi HJ, Bos EH, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry 2011;33:203-16. [Crossref] [PubMed]
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993;270:1819-25. [Crossref] [PubMed]
- Connerney I, Shapiro PA, McLaughlin JS, et al. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001;358:1766-71. [Crossref] [PubMed]
- Joint Formulary Committee. British National Formulary London. Available online: http://www.medicinescomplete.com
- Javors MA, Houston JP, Tekell JL, et al. Reduction of platelet serotonin content in depressed patients treated with either paroxetine or desipramine. Int J Neuropsychopharmacol 2000;3:229-35. [Crossref] [PubMed]
- Hergovich N, Aigner M, Eichler HG, et al. Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clin Pharmacol Ther 2000;68:435-42. [Crossref] [PubMed]
- Spiess BD. Transfusion of blood products affects outcome in cardiac surgery. Semin Cardiothorac Vasc Anesth 2004;8:267-81. [Crossref] [PubMed]
- Engoren MC, Habib RH, Zacharias A, et al. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg 2002;74:1180-6. [Crossref] [PubMed]
- Xiong GL, Jiang W, Clare R, et al. Prognosis of patients taking selective serotonin reuptake inhibitors before coronary artery bypass grafting. Am J Cardiol 2006;98:42-7. [Crossref] [PubMed]
- Xiong GL, Jiang W, Clare RM, et al. Safety of selective serotonin reuptake inhibitor use prior to coronary artery bypass grafting. Clin Cardiol 2010;33:E94-8. [Crossref] [PubMed]
- Kim DH, Daskalakis C, Whellan DJ, et al. Safety of selective serotonin reuptake inhibitor in adults undergoing coronary artery bypass grafting. Am J Cardiol 2009;103:1391-5. [Crossref] [PubMed]
- Andreasen JJ, Riis A, Hjortdal VE, et al. Effect of selective serotonin reuptake inhibitors on requirement for allogeneic red blood cell transfusion following coronary artery bypass surgery. Am J Cardiovasc Drugs 2006;6:243-50. [Crossref] [PubMed]
- Tully PJ, Cardinal T, Bennetts JS, et al. Selective serotonin reuptake inhibitors, venlafaxine and duloxetine are associated with in hospital morbidity but not bleeding or late mortality after coronary artery bypass graft surgery. Heart Lung Circ 2012;21:206-14. [Crossref] [PubMed]
- Gagne JJ, Polinski JM, Rassen JA, et al. Selective Serotonin Reuptake Inhibitor Use and Perioperative Bleeding and Mortality in Patients Undergoing Coronary Artery Bypass Grafting: A Cohort Study. Drug Saf 2015;38:1075-82. [Crossref] [PubMed]
- Chocron S, Vandel P, Durst C, et al. Antidepressant therapy in patients undergoing coronary artery bypass grafting: the MOTIV-CABG trial. Ann Thorac Surg 2013;95:1609-18. [Crossref] [PubMed]
- Sajan F, Conte JV, Tamargo RJ, et al. Association of Selective Serotonin Reuptake Inhibitors with Transfusion in Surgical Patients. Anesth Analg 2016;123:21-8. [Crossref] [PubMed]
- Ghassemi M, Marshall J, Singh N, et al. Leveraging a critical care database: selective serotonin reuptake inhibitor use prior to ICU admission is associated with increased hospital mortality. Chest 2014;145:745-52. [Crossref] [PubMed]
- Hata M, Yagi Y, Sezai A, et al. Efficacy of prophylactic treatment with selective serotonin reuptake inhibitors for depression after open-heart surgery. Surg Today 2011;41:791-4. [Crossref] [PubMed]
- Monster TB, Johnsen SP, Olsen ML, et al. Antidepressants and risk of first-time hospitalisation for myocardial infarction: a population-based case-control study. Am J Med 2004;117:732-7. [Crossref] [PubMed]
- Cooper TA, Valcour VG, Gibbons RB, et al. Spontaneous ecchymoses due to paroxetine administration. Am J Med 1998;104:197-8. [Crossref] [PubMed]
- Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003;108:939-44. [Crossref] [PubMed]
- Serebruany VL, Suckow RF, Cooper TB, et al. Relationship between release of platelet/endothelial biomarkers and plasma levels of sertraline and N-desmethylsertraline in acute coronary syndrome patients receiving SSRI treatment for depression. Am J Psychiatry 2005;162:1165-70. [Crossref] [PubMed]
- de Abajo FJ, Rodríguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ 1999;319:1106-9. [Crossref] [PubMed]
- Dalton SO, Johansen C, Mellemkjaer L, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med 2003;163:59-64. [Crossref] [PubMed]
- Movig KL, Janssen MW, de Waal Malefijt J, et al. Relationship of serotonergic antidepressants and need for blood transfusion in orthopedic surgical patients. Arch Intern Med 2003;163:2354-8. [Crossref] [PubMed]