Kawasaki disease: multiple giant coronary aneurysms intervention and pacemaker implantation due to complete heart block—a case report
Introduction
Coronary artery aneurysms (CAAs) are the main complication of Kawasaki disease (KD) that cause myocardial infarction and cardiovascular collapse. While early high-dose intravenous immunoglobulin (IVIG) can reduce the incidence of CAAs, there is no agreement on the effective treatment of CAAs in the acute or subacute stage of KD, especially for large or giant CAAs which could lead to cardiovascular collapse. This case report is of the presentation of a 19-month-old girl who developed multiple CAAs and myocardial infarction which further complicated persistent total heart block after delayed diagnosis of KS. A work-up to revascularize right coronary and restore the heart rate failed. To the best of our knowledge, this is the first case report of urgent percutaneous coronary intervention (PCI) intervention for CAAs and complete heart block after KS.
Case presentation
Initial presentation to the clinic
On June 12, 2016, a 19-month-old girl presented to a local clinic in Hebei province (in the middle of China) complaining of fever (with highest temperature of 39.5 °C), red eyes, and swollen lips but no tongue abnormalities and no body rash. She was treated with systematic antibiotics for 12 days without benefit.
Presentation at a local hospital
On June 25, 2016, the girl was transferred to a local hospital. Blood analysis showed elevated white blood cells (WBC) 24.2×10−9/L (neutrophil granulocyte 75.9% and lymphocyte 15.9%), platelet count (PLT) 397×10−9/L, and erythrocyte sedimentation rate 45 mm/h. Echocardiogram (UCG) revealed left main coronary artery dilated to 6.1 mm and proximal right coronary artery (RCA) dilated to 7 mm. Both atrial and ventricular size was normal. Left ventricular end-diastolic diameter was 26 mm and left ventricular ejection fraction (LVEF) was 61%. No significant regurgitation was identified. The girl was diagnosed to have KD on day 13 of her illness and received IVIG, anticoagulants, adjunctive therapy, and antibiotics. Medications are listed in Table 1. Her body temperature subsided for a very short time and soon fluctuated between 37.2 and 38.8 °C. On June 30, lip cyanosis emerged. On July 11, her heart rate had decreased to 30–70 beats/min. Electrocardiogram (ECG) revealed bradycardia, acute inferior myocardial infarction, and complete heart block. Intravenous infusion of 0.3 mg atropine (0.03 mg/kg) failed to increase heart rate. Myocardial enzymes were elevated showing myoglobin 878 ng/mL, troponin I 0.81 ng/mL, and creatinine kinase isozyme 14.5 ng/mL. Leukocytosis (WBC 24.9×10−9/L) and thrombocytosis (PLT 535×10−9/L) were identified.

Full table
Patient transferred to General Hospital of PLA army in Beijing
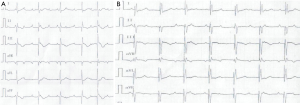
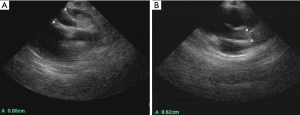
On July 12, 2016, the girl was transferred to our hospital for further evaluation and treatment. Physical examination revealed temperature 37.7 °C, pulse rate 60 beats/minute, respiratory rate 30/minute, blood pressure 70/45 mmHg, and body weight 10 kg. There was desquamation on palms and feet, lip cyanosis, injected throat but no tongue abnormalities, and no body rash. Liver was soft and there was an enlarged edge under the costal region. Myocardial enzymes increased significantly: myoglobin 1,665 ng/mL, troponin I 50 ng/mL, and creatinine kinase isozyme 171 ng/mL. Brain natriuretic peptide increased to 595 pg/mL, serum glutamic oxaloacetic transaminase was 5,017 U/L and serum glutamic pyruvic transaminase was 3,333 U/L. Blood uric acid was 707 umol/L and C-reactive protein was 5.9 mg/L. ECG revealed bradycardia (60 beats/minute), ST segment elevation in leads II, III, and AVF, abnormal Q wave, third degree heart block, and junctional escape rhythm (Figure 1A,B). Chest X-ray revealed enlarged cardiac silhouette. UCG showed aneurysms in left anterior descending and right coronary arteries (LAD) (Figure 2A,B), abnormal movement of the left ventricular wall and interventricular septum, mild regurgitation of mitral valve and tricuspid valve. The maximum diameter of proximal RCA dilated to 8.8 mm (Figure 2A). Left ventricular end-diastolic diameter was 32 mm and LVEF was 50%. KD was confirmed with concomitant acute inferior wall myocardial infarction, myocarditis, third degree heart block, bradycardia, and acute liver injury. The patient improved following treatment with antibiotics and anticoagulants (Table 1 shows the detail of medications). Body temperature fell to normal, blood inflammatory factors declined significantly, but lip cyanosis persisted.
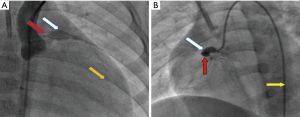
On July 19, coronary angiography revealed a giant aneurysmal dilatation (about 10 mm) in the LAD and a mild stenosis at the distal end of the aneurysm with TIMI-3 flow (see Figure 3A). Lesion position differed from that of the UCG study of the local hospital which showed left main CAA with diameter of about 10 mm. Another giant aneurysmal dilatation was formed at the proximal end of RCA with a diameter of about 8 mm and with 100% occlusion at the first flexure with TIMI-0 flow (see Figure 3B). Left circumflex artery was normal. Due to the persistent bradycardia and the mild stenosis on LAD, PCI was performed on RCA. But 6F JR4.0 guide catheter with NS guide wire failed to pass the occluded lesion, fielder guide wire with microcatheter assistance also failed. Multiple attempts with Pilot 50 guide wire passed the occlusion eventually, but the procedure was stopped due to dissection in the aneurysm. Because bradycardia persisted (with heart rate between 30–60 beats/min at rest and 70–80 beats/min after continuous isoprenaline intravenous infusion with a pump), a single chamber permanent cardiac pacemaker (Medtronic Res Ro1 VVI) was implanted successfully on August 9th, 2016. A double chamber pacemaker is anticipated to replace the single chamber one later when the patient grows up. The pacemaker was set at 80 beats/minute (Figure 1A,B). Lip cyanosis disappeared and the patient remained stable until she was discharged one week later after the pacemaker was implanted. At discharge aspirin was prescribed at 40 mg/day to prevent coronary thrombosis.
Follow up
The patient was found to be doing well at 3, 6, and 12 months follow-up based on ECG and UGG. The pacemaker rate was adjusted to 90 beats per minute at 3 months follow-up. UCG showed all coronary aneurysms had disappeared at 12 months follow-up.
Discussion
Our case highlights a critical point regarding treatment of large (6–8 mm) or giant aneurysms (>8 mm) in KD in the acute or subacute stage especially when the lesions endanger cardiac function. We were not able to recanalize the stenotic dissection lesion after many attempts with PCI. PCI is not recommended routinely for coronary thrombosis and coronary occlusion in KD treatment and has been performed in only a limited number of cases in the late cardiovascular sequelae of childhood KD (1). Not only is the vasculopathy of coronary lesions in KD very different from the pathology of typical coronary atherosclerosis but the diameter of coronary artery in very young children is also much narrower than those in adults. There are currently no established criteria for use of PCI in acute or subacute stenotic aneurysmal lesions, and the safety and long-term outcomes of PCI also remain undefined in acute and subacute stage of KD.
Bradycardia is rarely reported as a complication of KD following myocardial infarction. Yamamoto (2) studied a series of 155 patients and only one patient developed bradycardia. Our patient had persistent complete heart block and there was no response to atropine or isoprenaline. Coronary angiography revealed complete occlusion of the right proximal coronary artery. A pacemaker had to be placed because of the failure of RCA revascularization.
Valve regurgitation is another severe complication which mainly results from papillary muscle dysfunction and myocardial infarction. Kato (3) studied 594 KD patients and found the incidence of mitral regurgitation in acute and subacute stage was 1%. Our patient developed mitral and tricuspid regurgitation which is tandem to large areas of aneurysmal obstructive lesions. CAA expanded dramatically within 2 weeks from 6 to 10 mm in LAD, and from 7 to 8 mm in RCA. Cardiac function was not able to compensate, and heart insufficiency occurred which might have caused lip cyanosis.
Conclusions
Coronary arteries can be damaged permanently in acute and subacute stages of KD. Our unique case reemphasizes the narrow window for effective IVIG regimen to prevent or to stop the progression of coronary damage. There is urgent need to address the intervention to reduce coronary damage, possibly with PCI, when patients fail IVIG treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the parents of the patient to publish the case study in JTD before data collection.
References
- McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017;135:e927-e99. [Crossref] [PubMed]
- Yamamoto LG, Martin JE. Kawasaki syndrome in the ED. Am J Emerg Med 1994;12:178-82. [Crossref] [PubMed]
- Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996;94:1379-85. [Crossref] [PubMed]


