3D-printing aided resection of intratracheal adenoid cystic carcinoma and mediastinal mature cystic teratoma in a 26-year-old female: a case report
Introduction
Although adenoid cystic carcinoma (ACC) ranks 2nd of the common histologic types, primary neoplasms of trachea only comprises around 0.2% of all respiratory carcinoma (1-3). Thus only a few centers have abundant experience, according to which, complete surgical resection remains the ideal treatment. However, resectable length of trachea should be controlled precisely for its non-renewability and poor flexibility, calling for careful preoperative evaluation. Especially for those intratracheal tumors without extension out of trachea, the localization could be quite difficult. Furthermore, if there is an anterior superior mediastinal teratoma (MT) involving the aorta besides the intratracheal tumor, operation could become much more complicated. On the other hand, 3D-printing has been well accepted and widely used in the field of surgery in recent years, making complicated surgeries much more precise and efficiency. As far as we know, here we report the first case of 3D-printing-aided resection of intratracheal ACC and anterior superior MT in one operation.
Case presentation
A 26-year-old female with dyspnea and dry cough for 2 months was transferred to our center and physical examination revealed inspiratory stridor without cyanosis. Bronchoscopy found an 11 mm × 13 mm quasi-circular mass with broad base in the left wall of thoracic trachea, causing 90% obstruction, and pathology of biopsy suggested ACC. However, the subsequent CT scan with contrast found not only ACC, but also a circular 20 mm-diameter occupying lesion in the anterosuperior mediastinum, indicating MT. The patient was given bronchodilator and oxygen before surgery, but her symptoms weren’t relieved.
After the consultation of multi-disciplinary team for tracheal malignancy, including the experts of thoracic surgery, anesthesiology, respiratory medicine, medical imaging and pathology, it has been decided to remove the two tumors in one operation. Given that the resectable length of trachea should be controlled under 4 cm as the patient was a 158 cm-tall female, the localization of the ACC was key to the success to the operation. But we found it difficult to locate the ACC precisely during operation under direct vision as it hadn’t invaded out of trachea. In this way, 3D-printing was used to facilitate surgical planning and simulation practice (Figure 1A). After we got the 3D-printed model, surgical approaches were evaluated by rehearsal, and the median sternotomy was finally selected rather than lateral thoracotomy. In addition, according to the model, the outside of the trachea was smooth without ACC lesion, and the MT grew close to the ascending aorta and the ACC grew behind the brachiocephalic artery, in which case, artery reconstruction might be performed. Therefore, the MT was planned to be resected first, and then brachiocephalic artery was explored to locate the ACC for guiding resection by measuring the distance between the artery and the dissected margins.
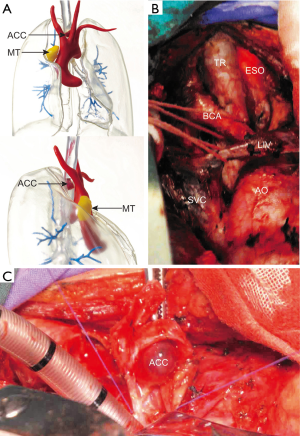
During operation, we performed median sternotomy, MT removal, left common carotid artery exploring without reconstruction, trachea with ACC resection and end-to-end trachea anastomosis in due order as planned (Figure 1B). Three tracheal rings with tumor lesion (Figure 1C) and a 20 mm-diameter circular mass with surrounding thymic tissue were resected as expected without too much hesitation, which shortened the duration of anesthesia, and pathologically diagnosed as ACC with negative margins and mature cystic teratoma respectively, according to which, she was classified as T1N0M0 trachea ACC based on Bhattacharyya’s staging system (4).
After surgery, she was extubated immediately, and her symptoms completely vanished. Additionally, a neck-holding position for tension-free anastomosis recovery was enforced by a self-designed jacket instead of Grillo stitch. She was given antibiotics, ultrasonic nebulization and fluid support routinely. On post-operative day 7, she was discharged without complications. According to the latest follow-up of 6 months after surgery, she has achieved full recovery and restarted her career as a local shopkeeper.
Discussion
About a century ago, ACC used to be named as ‘cylindroma’ by Billroth with low malignant potential, which only accounts for about 10% of tracheal malignancies. The patients with tracheal ACC usually present with the symptoms of upper airway obstruction such as coughing and wheezing and physical examination reveals inspiratory stridor, as our case. Submucosal or perineural invasion, direct extension and hematogenous dissemination are the common ways of its spreading (5), and it rarely spread through lymphatic system.
According to the studies of long-term follow-up, radical resection is still regarded as the best treatment for primary tracheal malignancies, including ACC (6). In some cases, it might be difficult to obtain negative margins because of the limit in resection length, but a few researchers tended to believe that the differences between the overall survival rates of patients with complete and incomplete resection seemed not to be not significant (7,8). For those inoperable patients, radiotherapy remains to be the first-line treatment (9). Though some other forms of treatment such as new types of radiotherapy and endoscopic resection (10,11) have been invented and practiced, their long-term outcomes compared with that of traditional resection remains unclear because of its low morbidity. Currently, Doggett S et al have reported that 3 patients with tracheal ACC were implanted with 103Pd under CT-fluoroscopic guidance utilizing percutaneous approach and responded well in the short-term with no chronic side effects (12). Therefore, more comparing studies should be encouraged to apply evidence for decision making.
In recent years, 3D-printing has been used to aid trachea surgery planning and airway evaluation in individual cases with complicated situations, which has achieved satisfying results (13-15) by making operation safer and easier, showing a great potential in the era of precision medicine. Taking the case above as an example, with the help of 3D-printed model, the safest approach could be confirmed by rehearsal, and the localization of the lesion became much easier and more accurate, which saved the patients plenty of time and avoided too much disturbance or damage of the surrounding organs.
In the field of trachea surgery, the limit of resectable length has always been the biggest obstacle. Nowadays, several different kinds of 3D-printed tracheal scaffolds have been invented for reconstruction, which has showed promising short-term outcomes, but precisely suitable artificial trachea without rejection might solve the problem thoroughly. It has been illuminated that the 3D-printed ring reinforcements of polycaprolactone and poly-lactic-co-glycolic acid showed improved mechanical properties (16). Recently, Rehmani et al. has reported the implantation of 3D-printed bioengineered tracheal grafts in animals with satisfied short-term and mid-term results, which was made of polycaprolactone and extracellular matrix in animals with satisfied short-term and mid-term results (17), encouraging thoracic surgeons to focus more on the reconstruction rather than destruction. Therefore, the combination of tissue engineering and 3D-printing would provide more possibilities for tracheal reconstruction in the future.
In conclusion, here we report a rare case diagnosed with intratracheal ACC and mediastinal mature cystic teratoma at the same time and the following surgical treatment with the help of 3D printing.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: We have obtained the patient’s signed consent form for publication of all the details of her case in journals without showing her name and face, which is available from the corresponding author on reasonable request.
References
- Nouraei SM, Middleton SE, Nouraei SA, et al. Management and prognosis of primary tracheal cancer: a national analysis. Laryngoscope 2014;124:145-50. [Crossref] [PubMed]
- Junker K. Pathology of tracheal tumors. Thorac Surg Clin 2014;24:7-11. [Crossref] [PubMed]
- Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol 2011;34:32-7. [Crossref] [PubMed]
- Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg 2004;131:639-42. [Crossref] [PubMed]
- Le Péchoux C, Baldeyrou P, Ferreira I, et al. Thoracic adenoid cystic carcinomas. Cancer Radiother 2005;9:358-61. [PubMed]
- Yang H, Yao F, Tantai J, et al. Resected Tracheal Adenoid Cystic Carcinoma: Improvements in Outcome at a Single Institution. Ann Thorac Surg 2016;101:294-300. [Crossref] [PubMed]
- Kaminski JM, Langer CJ, Movsas B. The role of radiation therapy and chemotherapy in the management of airway tumors other than small-cell carcinoma and non-small-cell carcinoma. Chest Surg Clin N Am 2003;13:149-67. [Crossref] [PubMed]
- Bittner N, Koh WJ, Laramore GE, et al. Treatment of locally advanced adenoid cystic carcinoma of the trachea with neutron radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:410-4. [Crossref] [PubMed]
- Webb BD, Walsh GL, Roberts DB, et al. Primary tracheal malignant neoplasms: the University of Texas MD Anderson Cancer Center experience. J Am Coll Surg 2006;202:237-46. [Crossref] [PubMed]
- Miller RJ, Murgu SD. Bronchoscopic resection of an exophytic endoluminal tracheal mass. Ann Am Thorac Soc 2013;10:697-700. [Crossref] [PubMed]
- Napieralska A, Miszczyk L, Blamek S. Tracheal cancer - treatment results, prognostic factors and incidence of other neoplasms. Radiol Oncol 2016;50:409-17. [Crossref] [PubMed]
- Doggett S, Chino S, Lempert T, et al. Percutaneous CT-fluoroscopic-guided radioisotope seed placement for the management of adenoid cystic carcinoma of the trachea. Brachytherapy 2017;16:639-45. [Crossref] [PubMed]
- Wilson CA, Arthurs OJ, Black AE, et al. Printed three-dimensional airway model assists planning of single-lung ventilation in a small child. Br J Anaesth 2015;115:616-20. [Crossref] [PubMed]
- Han B, Liu Y, Zhang X, et al. Three-dimensional printing as an aid to airway evaluation after tracheotomy in a patient with laryngeal carcinoma. BMC Anesthesiol 2016;16:6. [Crossref] [PubMed]
- Tam MD, Laycock SD, Jayne D, et al. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep 2013;7:34-43. [Crossref] [PubMed]
- Ott LM, Zabel TA, Walker NK, et al. Mechanical evaluation of gradient electrospun scaffolds with 3D printed ring reinforcements for tracheal defect repair. Biomed Mater 2016;11:025020. [Crossref] [PubMed]
- Rehmani SS, Al-Ayoubi AM, Ayub A, et al. Three-Dimensional-Printed Bioengineered Tracheal Grafts: Preclinical Results and Potential for Human Use. Ann Thorac Surg 2017;104:998-1004. [Crossref] [PubMed]


