Risk factors and methylenetetrahydrofolate reductase gene in congenital heart disease
Introduction
Congenital heart disease (CHD) is one of the most common birth defects, with an incidence of 28 per 100 congenital defects in China (1). CHD involves malformation of the cardiovascular system during the embryo stage (2). CHD is a multifactorial disease; its etiology is not fully understood. The occurrence of CHD might be affected by both genetic factors and environmental factors (3). Polymorphisms in genes associated with one-carbon metabolism are risk factors for heart disease (4). The methylenetetrahydrofolate reductase (MTHFR) gene 677 C>T variant is the best-characterized polymorphism in this regard, and this gene has become a research hotspot for understanding genetic susceptibility factors for CHD. Additionally, a variety of potential environmental risk factors, such as parental age, race, occupation and exposure to chemical agents, have been proposed (5-7). Gender prevalence has been completed in CHD children (8). However, few studies have reported on interactions between genetic factors and environmental factors in CHD (9).
In this study, we explored the impact of different environmental factors on CHD prevalence. Furthermore, we identified MTHFR gene polymorphisms in children with CHD and their parents as well as in a control cohort. Finally, we evaluated interactions between MTHFR gene polymorphisms and environmental risk factors in CHD.
Methods
Subjects
From May 2013 to May 2014, 346 children with CHD and their parents were recruited from Zhengzhou Children’s Hospital, which is in the Henan Province of China. All patients were diagnosed according to standard clinical criteria and ultrasonographic examination. The types of CHD included ventricular septal defect (VSD), atrial septal defect (ASD), tetralogy of Fallot (TF), double-outlet right ventricle (DORV), patent ductus arteriosus (PDA) and compound CHD (two or more than two simple types of CHD combined). As a control, 237 healthy families were recruited. The control children included 178 children who had upper respiratory tract infection and 59 children who had fractures. They had no occurrence of CHD and the absence of any chronical diseases. All children came from the same region. The average age of the children with CHD was 6.45±2.24 years. Males comprised 51% of the children with CHD. The average age of the control children was 6.15±2.31 years. The percent of the male sex in these children was 54%. There was no statistical significance (P>0.05). Both the case and control parents were interviewed face-to-face to collect information such as age, detailed medical history during pregnancy, family history and lifestyle exposures. Each participant donated 3 mL of venous blood for DNA genotyping.
This study was approved by the Ethics Review Committee of the Henan Institute of Population and Family Planning in Zhengzhou, China (No. 2012CQ001). Written informed consent was obtained from all the adult participants and guardians on behalf of the children enrolled in the study.
Measurements
Genomic DNA was isolated from whole peripheral blood with a QIAamp DNA Mini Kit (QIAGEN GmbH, Hilden, Germany) and stored at ‒20 °C.
To determine the genotype of the MTHFR gene, the genomic DNA was amplified by specific primers using PCR. The MTHFR C677T primer sequences (10) are as follows: forward, 5'-TGAAGGAGAAGGTGTCTGCGGGA-3' and reverse, 5'-AGGACGGTGCGGTGAGAGTG-3'. The PCR amplifications were performed in a total volume of 25 µL, which consisted of 10 pmol of each primer, MasterMix and template DNA. Thermal cycling conditions were 94 °C for 5 min, followed by 30 cycles at 94 °C for 30 s, 58 °C for 45 s and 72 °C for 45 s, and a final extension at 72 °C for 5 min. The PCR products were digested with 2–4 units of restriction enzyme, Hinf I at 37 °C for 16 h in 10× Fast Digest Green Buffer at a final volume of 30 µL. The restriction patterns of the PCR products were determined via separation on 2–4% agarose gels. The agarose gels were stained in ethidium bromide and visualized using an Alpha InnoTech imager. The PCR products were also submitted for sequencing at the Sangon Biotechnology Limited Company.
Data analysis
Data analysis was performed using SPSS version 18.00 (SPSS Inc, South Wacker Drive, Chicago, IL, USA). Alleles and genotype distributions were tested for deviation from the Hardy-Weinberg equilibrium using a Chi-square test. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were used to illustrate associations, with P<0.05 considered statistically significant in all tests. Three measures of biological interaction were used (11): RERI, the relative excess risk due to interaction; AP, the attributable proportion due to interaction; and S, the synergy index. If no biological interaction exists, RERI and AP are equal to 0, and S is equal to 1.
Results
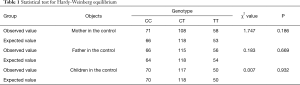
Table 1 shows genotype frequencies of the control. There was no significant difference from the expected value, with all of the P>0.05, which showed that the genotype frequencies of the control were coincident with the Hardy-Weinberg genetic equilibrium.
Full table
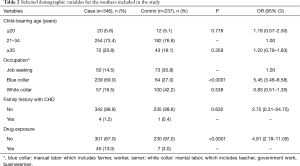
Table 2 shows selected maternal characteristics from the CHD and control groups. There were significant differences in the occupational statuses of the mothers and their levels of drug exposure during gestation between the controls and the cases (P<0.05 for both comparisons). These two variables significantly increased offspring CHD risk. If a mother had a blue-collar occupation, her child’s CHD risk was increased (OR =5.45, 95% CI: 3.46–8.58), whereas a white-collar occupation decreased this risk (OR =0.83, 95% CI: 0.51–1.35) compared to a job-seeking status. Drug exposure during gestation also increased CHD risk (OR =4.91, 95% CI: 2.18–11.09).
Full table
Table 3 shows the genotype distributions for the MTHFR rs1801131 variant between the mothers of the children with CHD and those of the control children. The frequency of the MTHFR gene 677 TT variant significantly differed between the mothers of the children with CHD and those of the controls (CC, 14.4%; CT, 45.1%; TT, 40.5% for the mothers in the case group; CC, 30.0%; CT, 45.6%; TT, 24.4% for the mothers in the control group). Table 4 shows the genotype distributions for the MTHFR rs1801131 variant among the fathers of the children with CHD and the controls. The frequencies of genotypic variants in the MTHFR gene did not significantly differ between the fathers of the children with CHD and those of the controls (CC, 29.0%; CT, 44.5%; TT, 26.5% for the fathers in the case group; CC, 27.8%; CT, 48.6%; TT, 23.6% for the fathers in the control group).

Full table

Full table
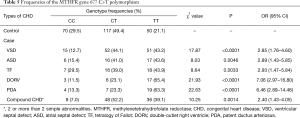
Table 5 shows the frequencies of the MTHFR gene 677 C>T polymorphism in the children with CHD. Notably, the frequency of the TT genotype was significantly higher in the children with CHD than in the control group (P<0.05).
Full table
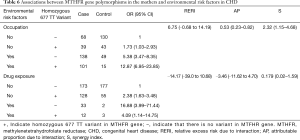
Table 6 shows the identified interactions between MTHFR gene polymorphisms in the mothers and environmental risk factors in CHD. No biological interaction was found between the presence of a homozygous 677 TT variant and a mother’s occupational status (RERI =6.75, CI: ‒0.68 to 14.19) or drug exposure levels during gestation (RERI =‒14.17, CI: ‒39.0 to 10.68).
Full table
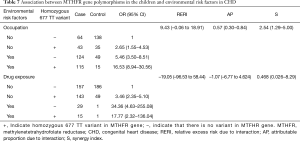
Table 7 shows the identified interactions between MTHFR gene polymorphisms in the children and environmental risk factors in CHD. No biological interaction was found between the presence of a homozygous 677 TT variant in a child and the mother’s drug exposure levels during gestation (RERI =‒19.05, CI: ‒96.53 to 58.44). There was an interaction between the presence of a homozygous 677 TT variant in a child and their mother’s occupational status (RERI =9.43, CI: ‒0.06 to 18.91).
Full table
Discussion
In this study, we investigated four main risk factors in mothers of children with CHD in China: childbearing age, occupational status, family history of CHD and drug exposure during gestation. Neither a mother's childbearing age nor her family history of CHD had a significant association with CHD susceptibility. Associations were observed between CHD prevalence and a mother's occupational status or her exposure to drugs during gestation. Most drugs that led to gestational exposure were associated with treating fever and influenza during early pregnancy (less than 12 weeks of gestation). However, because of this early time point, most of the mothers could not remember what specific drugs they had taken. Additionally, the mothers in both groups had limited education and therefore had difficulties in describing their childbearing histories. However, our findings suggested that a mother’s social status and living conditions might affect the incidence of CHD in their children to some extent. This means that blue-collar occupation was associated with an increased risk of CHD. Some studies had shown that their environment during work was more frequently exposed to toxic substances (12). Meanwhile, their diet and nutritional status were not rich and irregular, which might influence the development of the heart (13).
There is a contradictory conclusion that gender differs in CHD. Dr. Samanek’s research (8) found gender differences in CHD children. However, our results, as well as Dr. Wang’s (14) and Dr. van Beynum’s results (15), had shown that there was no difference. In fact, Dr. Samanek’s research analyzed gender differences according to different types of CHD. We analyzed gender differences between CHD children and healthy children. Thus, these results were not controversial.
MTHFR is a key enzyme in the methionine cycle and can regulate homocysteine levels. Single nucleotide mutations in the MTHFR gene can decrease the enzyme’s activity. To date, four single nucleotide polymorphisms (203 G→A, 677 C→T, 1286 A→C, and 1793 G→A) have been identified in the MTHFR gene. The 677 C→T variant has been shown to decrease MTHFR’s enzyme activity and increase plasma levels of homocysteine (16). The homozygous 677 TT variant and the heterozygous 677 CT variant have approximately 30% and 70% of residual enzyme activity, respectively, compared to the wild-type 677 CC variant. Racial differences and regional differences in genetic MTHFR polymorphisms are distinct. The population in Henan Province is the largest in China; our research is a useful supplement to previous research. In the current study, the frequency of the homozygous 677 TT variant in the mothers in the case group was significantly higher than in the mothers in the control group. This demonstrated that the homozygous 677 TT variant of the MTHFR gene in the mothers with CHD and in the children with CHD increased the risk of CHD. In contrast, we also found that the MTHRF gene did not differ between the fathers of the children with CHD and those of the controls. It is surmised that a neural tube defect during organogenesis in the maternal MTHFR genotype plays a role in fetal folate bioavailability (17), but the paternal MTHFR gene has no contribution. The frequency of the homozygous 677 TT variant was significantly higher in the children with CHD than in the control group, suggesting an association between the 677 C→T mutation in the MTHFR gene and the prevalence of CHD. This result is inconsistent with previous research reports by Hobbs (18). One difference between our study and the Hobbs study is that 80% of the mothers in the latter research reported taking either folic acid or a multivitamin containing folic acid during pregnancy. In contrast, in the current study, few mothers with the homozygous 677 TT variant regularly took a folate supplement during pregnancy. Therefore, it is likely that folic acid supplementation before and during early pregnancy could decrease the risk of fetal CHD and vice versa.
Some studies have shown significant differences in the incidence of the same disease among populations living in the same environment (19). Thus, both genetic factors and environmental factors interact to affect disease prevalence, including that of CHD. In the present research, we analyzed interactions between environmental factors (mother’s occupation and drug exposure during pregnancy) and MTHFR gene polymorphisms. We found a positive, significant, additive interaction between the presence of the homozygous 677 TT variant of the MTHFR gene in the children with CHD and the occupations of their mothers. Overall, the excess risk due to interactions between occupational exposure in the mothers and MTHFR gene mutations was 9.43 (RERI =9.43, CI: ‒0.06 to 18.91). Furthermore, the proportion of children with CHD affected by the interaction of these two risk factors was 57% (AP =57%, CI: 0.30–0.84). The CHD risk was increased by 2.54-fold (S =2.54, CI: 1.29–5.0) for mothers with occupational exposure who carried children homozygous for the 677 TT variant.
Conclusions
In conclusion, neither MTHFR gene polymorphisms nor environmental factors directly lead to CHD, but they can increase susceptibility to the disease, especially when they exist together. Our study indicates that screening high-risk populations for susceptibility and offering corresponding protection to them will decrease the prevalence of CHD.
Acknowledgements
Funding: This work was supported by a grant from the National Natural Science Foundation of China (No. 31471204).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Review Committee of the Henan Institute of Population and Family Planning in Zhengzhou, China (No. 2012CQ001). Written informed consent was obtained from all the adult participants and guardians on behalf of the children enrolled in the study.
References
- Dolk H, Loane M, Garne E. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation 2011;123:841-9. [Crossref] [PubMed]
- Liu CX, Shen AD, Li XF, et al. Association of TBX5 gene polymorphism with ventricular septal defect in the Chinese Han population. Chin Med J 2009;122:30-4. [PubMed]
- Zhu WL, Li Y, Yan L, et al. Maternal and offspring MTHFR gene C677T polymorphism as predictors of congenital atrial septal defect and patent ductus arteriosus. Mol Hum Reprod 2006;12:51-4. [Crossref] [PubMed]
- Cheng TY, Makar KW, Neuhouser ML, et al. Folate-mediated one-carbon metabolism genes and interactions with nutritional factors on colorectal cancer risk: Women's Health Initiative Observational Study. Cancer 2015;121:3684-91. [Crossref] [PubMed]
- Miller A, Riehle-Colarusso T, Siffel C, et al. Maternal age and prevalence of isolated congenital heart defects in an urban area of the United States. Am J Med Genet A 2011;155A:2137-45. [Crossref] [PubMed]
- Nembhard WN, Wang T, Loscalzo ML, et al. Variation in the prevalence of congenital heart defectsby maternal race/ethnicity and infant sex. J Pediatr 2010;156:259-64. [Crossref] [PubMed]
- Snijder CA, Vlot IJ, Burdorf A, et al. Congenital heart defects and parental occupational exposure to chemicals. Hum Reprod 2012;27:1510-7. [Crossref] [PubMed]
- Samanek M. Boy:girl ratio in children born with different forms of cardiac malformation: a populationbased study. Pediatr Cardiol 1994;15:53-7. [PubMed]
- Wyszynski DF, Correa-Villaseñor A, Graham TP. editors. Congenital heart defects. NEW York:, Oxford University Press, 2010.
- Lupo PJ, Goldmuntz E, Mitchell LE. Gene-gene interaction in the folate metabolic pathway and the risk of conotruncal heart defects. J Biomed Biotechnol 2010;2010:630940.
- Andersson T, Alfredsison L, Källberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575-9. [Crossref] [PubMed]
- Shi H, Yang S, Liu Y, et al. Study on environmental causes and SNPs of MTHFR, MS and CBS genes related to congenital heart disease. PLoS One 2015;10:e0128646. [Crossref] [PubMed]
- Elizabeth KE, Praveen SL, Preethi NR, et al. Folate, vitamin B12, homocysteine and polymorphisms in folate metabolizing genes in children with congenital heart disease and their mothers. Eur J Clin Nutr 2017;71:1437-41. [Crossref] [PubMed]
- Wang W, Wang Y, Gong F, et al. MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 Case-Control and TDT Studies. PLoS One 2013;8:e58041. [Crossref] [PubMed]
- van Beynum IM, den Heijer M, Blom HJ, et al. The MTHFR 677C->T polymorphism and the risk of congenital heart defects: a literature review and meta-analysis. QJM 2007;100:743-53. [Crossref] [PubMed]
- Frosst P, Blom H, Milos R. Identification of a candidate genetic risk factor for vascular disease: a common mutation in methylentetrahydrofolate reductase. Nat Genet 1995;10:111-3. [Crossref] [PubMed]
- Botto LD, Yang Q. 5,10-methylenetetrahydrofolate reductase gene variants and congenital anomalies: A HUGE review. Am J Epidemiol 2000;151:862-77. [Crossref] [PubMed]
- Hobbs CA, James SJ, Parsian A, et al. Congenital heart defects and genetic variants in the methylenetetrah- ydroflate reductase gene. J Med Genet 2006;43:162-6. [Crossref] [PubMed]
- Ji F, Xia S. The relationship between gene-environment interaction and human diseases. Fudan Univ J Med Sci 2007;34:935-8.


