Regenerative concepts in cardiovascular research: novel hybrid therapy for remodeling ischemic cardiomyopathy
Introduction
Treatment for coronary artery disease includes medical management, percutaneous coronary interventions (PCI), and coronary artery bypass grafting. However, despite optimal medical therapy and revascularization, ischemic cardiomyopathy is responsible for 538,000 deaths per year in the U.S. alone (1). Stem cell transplantation provides an opportunity to reduce mortality through its potential to fully heal and replace infarcted myocardium.
The role of mesenchymal stem cell (MSC) therapy in myocardial recovery
There is great potential for stem cells to improve cardiac remodeling and restore cardiac function, although a recent systematic review highlights the heterogeneity of results with stem cells in myocardial regeneration (2). Intracoronary injection of autologous bone marrow derived-mononuclear cells after PCI in patients with ST elevation myocardial infarction (STEMI), improves left ventricular ejection fraction (3), but this finding is not universally reported (4). Inconsistencies in methodologies, cell population use, and delivery methods likely contribute to these conflicting data.
MSCs are a reasonable candidate for cardiac stem cell transplantation. They can be easily isolated and grown over several passages and demonstrate directed multilineage differentiation. MSCs have decreased levels of MHC II expression compared to MHC I indicating low immunogenicity. This coupled with their immunosuppressive effects allow for successful allogenic transplantation.
While the mechanism of stem cell therapy remains uncertain, cardiac remodeling after stem cell transplantation is likely facilitated by paracrine activity to promote homing and differentiation into multiple cell types. Release of factors that inhibit inflammation and apoptosis, and increase angiogenesis further contributes to cardiac repair and functional improvement. In an in vitro model, MSCs secrete cytokines, antioxidants, and growth factors, including fibroblast growth factor (FGF)-2, hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) (5). FGF-2 reduces arrhythmias and apoptosis in ischemic cardiac tissue (6), while HGF and VEGF promote angiogenesis and improve hemodynamics in the damaged heart (7). HGF also mediates recruitment of progenitor cells to injury sites, augmenting repair and regeneration (8).
Over the past two decades, we have attempted stem cell transplantation in human and animal studies. In patients with acute myocardial infarction, intracoronary infusion of umbilical cord-derived MSCs improves left ventricle (LV) ejection fraction and limits LV dilatation 18 months after treatment (9). Infusion of allogenic bone marrow MSCs or autologous cardiosphere-derived cells (heart-derived progenitor cells expanded from myocardial biopsies) also improves LV recovery and limits adverse remodeling, as shown in the PROCHYMAL and CADUCEUS trials (4,10). Although the CADUCEUS trial showed a 12% reduction in infarct scar size and improved viability and regional wall function, the trial did not demonstrate significant improvement in global systolic function or quality of life.
Stem cell retention and survival
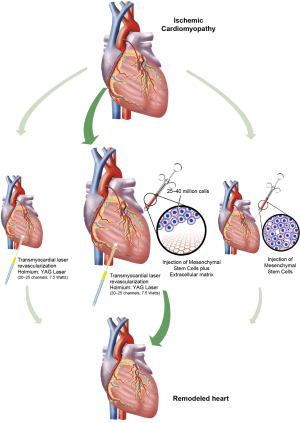
Stem cell retention and survival remains a challenge in a microenvironment of infarcted tissue. Preparation of stem cells prior to intracoronary or intramyocardial injection is important to enhance cell survival. Stem cells lose matrix attachments such as integrin molecules during preparation, which leads to apoptosis-mediated cell death. The hypoxic and pro-inflammatory microenvironment of infarcted myocardium also induces apoptotic signaling. Seeding of stem cells in bioscaffolds prior to transplantation, and pre-treatment of infarcted myocardium with transmyocardial laser revascularization (TMLR) or shockwave therapy are potential novel therapies to increase stem cell retention and survival in an ischemic microenvironment.
The role of bioscaffolds
Seeding of stem cells in three-dimensional structures of heart extracellular matrices (ECM) or ECM-like biomaterials can promote tissue repair and regeneration. The porous or fibrous preformed bioscaffolds are commonly used for cell delivery where cells are planted on patches before surgically attaching the patch to the epicardial surface. We have used organic acellular ECM derived from porcine small intestine mucosa as an interim bioscaffold onto which native cells can migrate and replace foreign ECM with permanent collagen and structural proteins to promote scar remodeling (11). 3D alginate scaffolds are made using hydrogel material derived from algae-based polysaccharides and can also be used to augment cell seeding and delivery. Cardiac patches with human MSC (hMSC) and rat type 1 collagen matrix show improvement in myocardial remodeling after infarction in a rat model (12). In this model, Simpson et al. observed a decrease in LV end systolic dimension and an increase in LV fractional shortening by 30%; however, hMSC were not detectable four weeks after patch application. Through modification of cytoskeletal structure and provision of a graftable milieu, bioscaffolds with ECM is a promising strategy for myocardial regeneration.
The role of transmyocardial laser revascularization
Since the 1980s, TMLR has been an option to treat refractory angina for patients who are not candidates for revascularization. TMLR is a surgical procedure where several channels are generated within the myocardium using a laser power source. Typically, 20-40 channels are created depending on the size of infarct. Currently, the FDA has approved two laser devices for surgical use: the carbon dioxide (CO2) (PLC Medical Systems, Franklin, MA, USA) and holmium:yttrium-aluminium-garnet (Ho:YAG) (CardioGenesis Corporation, Foothill Ranch, CA, USA) systems.
TMLR is thought to induce neovascularization, which may account for its long term clinical improvement and cardio-protection. Histological specimens from deceased patients who had undergone TMLR show a transformation from early necrosis and inflammation to fibrous scarring and increased capillary networks (13). This angiogenic effect is specific to laser systems that cause tissue injury, with sham- or excimer-lasers causing no vascular response in a swine model (14). In a recent meta-analysis, TMLR was shown to improve myocardial perfusion when assessed by PET imaging (15).
Because of the potential for angiogenesis with TMLR, we posit that TMLR may potentiate the effects of stem cells on myocardial recovery. In a rat infarct model, pre-treatment with TMLR prior to administration of MSCs increases angiogenesis and improves cardiac function (16). In a rat model of myocardial infarction, TMLR improves homing, engraftment and increases early cell survival of circulating MSCs (17). In humans, treatment with TMLR prior to intramyocardial stem cell injection is a feasible and safe technique and is associated with a reduction in ischemia and improved functional capacity (18).
The mechanism by which TMLR improves cell homing, engraftment and early survival is uncertain. TMLR may create a favorable microvascular environment for stem cells to survive once implanted into scar tissue. Further, the channel conduits may increase cell implantation prior to channel closure. Pro-survival cytokines and growth factors from TMLR induced inflammation zones may also enhance stem cell survival. TMLR alone directly contributes to myocardial regeneration through upregulation of specific transcription factors that enhance epithelial to mesenchymal transition (EMT) (19). Epicardial EMT promotes the formation of cardiac progenitor cells and lineage commitment. The result of this biological process is the production of endothelial cells, smooth muscle cells and even cardiomyocytes, all of which are involved in cardiac tissue repair and regeneration.
Overall, the regenerative capabilities of stem cells combined with TMLR appear synergistic for recovery and regeneration in the setting of ischemic cardiomyopathy.
The role of shockwave therapy
Low energy shockwaves are special acoustic waves that can be focused on specific regions of the body to cause local vasodilation angiogenesis (20). Myocardial shock wave therapy is delivered via extracorporeal cardiac shock waves (ECSW) or direct epicardial shock (DES). ECSW is a non-invasive treatment where acoustic waves are applied to selected areas of the heart under echocardiographic guidance. This process risks lung injury and is restricted to certain areas of the heart due to the narrow acoustic window to the heart. Alternatively, DES avoids lung complications and permits accessibility of the whole heart. The main limitation for DES is that it requires surgical access, and therefore is generally limited for use in patients undergoing cardiac surgery.
Clinical studies show shock wave therapy to be a safe and feasible option to provide symptomatic relief and increased myocardial perfusion in patients with refractory angina not amenable to revascularization (21). Shock wave therapy may also have some regenerative properties for the ischemic myocardium. In a porcine model of ischemic cardiomyopathy, shock waves improve ejection fraction, wall thickening fraction and regional myocardial blood flow of the ischemic region (20). In a rat model of ischemic cardiomyopathy, the application of a low-energy epicardial shock wave improves left ventricular ejection fraction, induces angiogenesis and causes a decline of N-terminal prohormone B-type natriuretic peptide (22). In human studies, extracorporeal shock wave therapy improves left ventricular ejection fraction and functional capacity in patients with ischemic cardiomyopathy (23). Shock wave therapy also improves SPECT stress and rest perfusion scans suggesting an angiogenic-mediated mechanism underlying the cardiac repair and regeneration (23). Although treatment with shockwaves in isolation for myocardial regeneration is encouraging, there is a paucity of data combining shockwave therapy with stem cell transplantation. This is an area that holds much promise and provides an exciting direction for future research.
The role of mechanical circulatory support in myocardial recovery
Mechanical circulatory support devices are a therapeutic option for patients with terminal heart failure and may provide a means for cardiac recovery (24). Technological improvements, miniaturization of next-generation devices, as well as minimally invasive implantation techniques have led to lower complication rates and improved long-term durability (25-28). In the clinical setting of decompensated heart failure, early reports suggest that mechanically unloading the heart provides a more favorable environment for stem cell engraftment (29).
Future directions
Research into regenerative therapies for heart failure has been largely frustrating, and a successful stem cell strategy remains elusive. New techniques to improve stem cell survival and retention may act to potentiate the regenerative properties of stem cells. The combination of hMSCs, bioscaffolds, and pre-treatment with TMLR or shockwaves may create the optimal environment for stem cell graft retention, angiogenesis, and myocardial regeneration (Figure 1). Early reports of its application in humans have been promising (30). In a field of cardiovascular research that has had few successes, we believe this hybrid therapy may pave the way for more promising results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016;133:447-54. [Crossref] [PubMed]
- Nguyen PK, Rhee JW, Wu JC. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol 2016;1:831-41. [Crossref] [PubMed]
- Wollert KC, Meyer GP, Muller-Ehmsen J, et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J 2017;38:2936-43. [Crossref] [PubMed]
- Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277-86. [Crossref] [PubMed]
- Schinköthe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev 2008;17:199-206. [Crossref] [PubMed]
- Nishida S, Nagamine H, Tanaka Y, et al. Protective effect of basic fibroblast growth factor against myocyte death and arrhythmias in acute myocardial infarction in rats. Circ J 2003;67:334-9. [Crossref] [PubMed]
- Estrada R, Li N, Sarojini H, et al. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol 2009;219:563-71. [Crossref] [PubMed]
- Neuss S, Becher E, Woltje M, et al. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004;22:405-14. [Crossref] [PubMed]
- Gao LR, Chen Y, Zhang NK, et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med 2015;13:162. [Crossref] [PubMed]
- Malliaras K, Makkar RR, Smith RR, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol 2014;63:110-22. [Crossref] [PubMed]
- Ferng A, Connell A, Nunez M, et al. Cardiac Regeneration in the Human Left Ventricle After CorMatrix Implantation. Ann Thorac Surg 2017;104:e239-41. [Crossref] [PubMed]
- Simpson D, Liu H, Fan T-HM, et al. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells 2007;25:2350-7. [Crossref] [PubMed]
- Gassler N, Wintzer HO, Stubbe HM, et al. Transmyocardial laser revascularization. Histological features in human nonresponder myocardium. Circulation 1997;95:371-5. [Crossref] [PubMed]
- Hughes GC, Kypson AP, Annex BH, et al. Induction of angiogenesis after TMR: a comparison of holmium: YAG, CO2, and excimer lasers. Ann Thorac Surg 2000;70:504-9. [Crossref] [PubMed]
- Iwanski J, Knapp SM, Avery R, et al. Clinical outcomes meta-analysis: measuring subendocardial perfusion and efficacy of transmyocardial laser revascularization with nuclear imaging. J Cardiothorac Surg 2017;12:37. [Crossref] [PubMed]
- Spiegelstein D, Kim C, Zhang Y, et al. Combined transmyocardial revascularization and cell-based angiogenic gene therapy increases transplanted cell survival. Am J Physiol Heart Circ Physiol 2007;293:H3311-6. [Crossref] [PubMed]
- Shahzad U, Li G, Zhang Y, et al. Transmyocardial revascularization induces mesenchymal stem cell engraftment in infarcted hearts. Ann Thorac Surg 2012;94:556-62. [Crossref] [PubMed]
- Dallan LAO, Gowdak LH, Lisboa LAF, et al. Cell therapy plus transmyocardial laser revascularization: a proposed alternative procedure for refractory angina. Rev Bras Cir Cardiovasc 2008;23:46-52. [Crossref] [PubMed]
- Germani A, Foglio E, Capogrossi MC, et al. Generation of cardiac progenitor cells through epicardial to mesenchymal transition. J Mol Med (Berl) 2015;93:735-48. [Crossref] [PubMed]
- Nishida T, Shimokawa H, Oi K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 2004;110:3055-61. [Crossref] [PubMed]
- Alunni G, Barbero U, Vairo A, et al. The beneficial effect of extracorporeal shockwave myocardial revascularization: Two years of follow-up. Cardiovasc Revasc Med 2017;18:572-6. [Crossref] [PubMed]
- Zimpfer D, Aharinejad S, Holfeld J, et al. Direct epicardial shock wave therapy improves ventricular function and induces angiogenesis in ischemic heart failure. J Thorac Cardiovasc Surg 2009;137:963-70. [Crossref] [PubMed]
- Fukumoto Y, Ito A, Uwatoku T, et al. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis 2006;17:63-70. [Crossref] [PubMed]
- Uribarri A, Rojas SV, Avsar M, et al. First series of mechanical circulatory support in non-compaction cardiomyopathy: Is LVAD implantation a safe alternative? Int J Cardiol 2015;197:128-32. [Crossref] [PubMed]
- Rojas SV, Avsar M, Hanke JS, et al. Minimally invasive ventricular assist device surgery. Artif Organs 2015;39:473-9. [Crossref] [PubMed]
- Rojas SV, Avsar M, Uribarri A, et al. A new era of ventricular assist device surgery: less invasive procedures. Minerva Chir 2015;70:63-8. [PubMed]
- Schmitto JD, Hanke JS, Rojas S, et al. Circulatory support exceeding five years with a continuous-flow left ventricular assist device for advanced heart failure patients. J Cardiothorac Surg 2015;10:107. [Crossref] [PubMed]
- Maltais S, Anwer LA, Tchantchaleishvili V, et al. Left Lateral Thoracotomy for Centrifugal Continuous-Flow Left Ventricular Assist Device Placement: An Analysis from the Mechanical Circulatory Support Research Network. ASAIO J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kazui T, Tran PL, Pilikian TR, et al. A dual therapy of off-pump temporary left ventricular extracorporeal device and amniotic stem cell for cardiogenic shock. J Cardiothorac Surg 2017;12:80. [Crossref] [PubMed]
- Avery RJ, Yu SK, Cherukuri G, et al. Remodeling Failing Human Myocardium with Hybrid Cell/Matrix and Transmyocardial Revascularization. ASAIO J 2017. [Epub ahead of print]. [Crossref] [PubMed]