Anatomic and surgical factors affecting the switch from minimally invasive transthoracic occlusion to open surgery during ventricular septal defect repair
Introduction
In recent years, under the guidance of echocardiography, the minimally invasive transthoracic occlusion procedure for ventricular septal defect (VSD) has been developed and has drawn increasing attention, achieving good clinical therapeutic effects (1,2). Echocardiography includes transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE). This study aimed to investigate the causes for patients switching from minimally invasive transthoracic closure of VSD to surgical repair under cardiopulmonary bypass (CPB) due to the failure of transthoracic closure. To do so, we retrospectively analyzed the findings from preoperative TTE, intraoperative TEE and surgical exploration among the 340 patients who underwent minimally invasive transthoracic closure of VSD in the past 2.5 years at our hospital.
Methods
Patients
We searched the electronic database of the patients in whom minimally invasive repair for VSD had been attempted between August, 2013 and May, 2016. The exclusion criteria were significant cardiac and non-cardiac comorbidities that could affect the clinical outcome or the cost of defect closure, evidence of significant heart failure at admission, and VSD with right-to-left shunting due to severe pulmonary hypertension.
A total of 340 VSD patients (178 males and 162 females) undergoing transthoracic closure under the guidance of TEE in the department of cardiac surgery of the First Affiliated Hospital of Guangzhou Medical University between August, 2013 and May, 2016 were included and retrospectively analyzed. The patients were 3 months to 33 years old and weighed 3.5 to 61 kg (mean =14.3±6.51 kg) (Table 1).

Full table
Ethics statement
In those suitable for both treatment options, the choice was made according to the preference of the patient and family. Informed consent was obtained from each patient or the legal guardian before either procedure. The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University (Number of approval: GYFYY-2013-17).
Equipment and methods
Equipment
The GE vivi-I and PHILIPS-iE Elite color Doppler ultrasonic diagnosis apparatus with transthoracic and transesophageal probes (for adult and children, respectively) were used. A nickel-titanium alloy wire weaving membranous occluder was obtained from Shanghai Shape Memory Alloy Material Co., Ltd. and LifeTech Scientific Corporation. The occluder was equilateral or eccentric with a mid-portion diameter of 4–12 cm. The closure disc was filled with five layers of polyester fiber membrane, which were capable of blocking the blood flow.
Anesthesia
The patients were anesthetized by combined intravenous inhalational anesthesia with tracheal intubation. After endotracheal intubation, the anesthesia machine (Drager Primas type) was connected for pressure-controlled ventilation.
Surgical procedures
The patients were placed in a supine position, and an esophageal l probe was inserted. The preoperative TTE was conducted to carefully assess the size of VSD, its position relative to the adjacent valves, and the presence of any other malformations and to preliminarily select the model of occluder umbrella. Under the guidance of TEE, an optimal puncture point that had the smallest angle with the VSD blood flow and the shortest distance from the VSD was selected. The vessels on the surface of the cardiac muscle were avoided. Meanwhile, the guide wire was passed through the VSD, and the location of the guide wire was estimated. The delivery sheath was established, and the left ventricular disc was sequentially released through the sheath. The transmission pole was slightly pulled back to make the left ventricular disc close to the interventricular septum. The right ventricular disc was released, and the push-pole was appropriately pushed to check the stability of the closure umbrella. The TEE was used for multi-planar inspections of the position and stability of closure umbrella, its interference on the adjacent valves, residual shunts and regurgitation. Attention should be paid to check whether there were continuous arrhythmias and conduction block. After repeated satisfactory examinations by TEE, the push-pole was released and was pulled out from the sheath, followed by hemostasis and closing the chest. After the operation, TEE multi-planar observation was continuously performed for 10–15 minutes.
Statistical analysis
Continuous data were expressed as the mean ± standard deviation (SD). Categorical data were indicated as number and percentage (numerator/denominator) and compared by Chi-square test or Fisher’s exact test, respectively. A P value less than 0.05 was deemed to indicate statistical significance of each test. All analyses were performed using IBM SPSS Version 20 (SPSS Statistics V20, IBM Corporation, Somers, New York).
Results
Among the 340 patients who underwent transthoracic closure of VSD, a total of 26 patients who were switched to CPB surgery were included in this study. Table 2 summarizes the cases switching to CPB surgery by year between 2013 and 2016. Table 3 indicated the switching cases by anatomic classification of VSD, but the overall difference did not reach significance (P=0.160). However, a marginal significance was observed in a comparison of the perimembranous and membranous aneurysm type: the perimembranous type had a lower switching rate than the membranous aneurysm type (6.22% vs. 13.10%) (P=0.061).

Full table

Full table
Patients were further stratified by surgery year. Table 4 summarizes the cases from 2013 to 2014 and Table 5 those of 2015 and 2016. Although the membranous aneurysm type had the highest rate of switching for both results, but no significant difference was found (all P>0.05).

Full table

Full table
Tables 6,7 demonstrate the causes for patient switching to CPB surgery according to the surgery time: Table 6 for 2013–2014 and Table 5 for 2015–2016, respectively. No significant differences were found in the distributions of causes (both P>0.05).

Full table

Full table
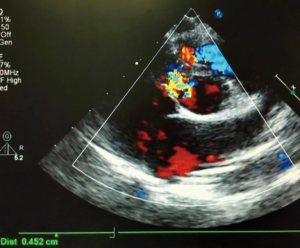
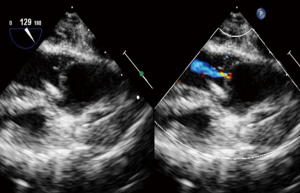
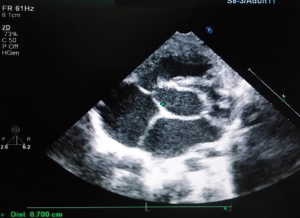
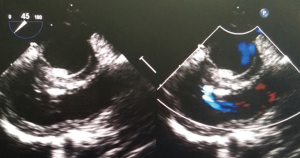
The preoperative TTE and intraoperative TEE are shown in Figures 1-4. Case 1 was a female, aged 7 years, who weighed 20 kg and was identified as No. 532194. The preoperative TTE showed 4.5 mm VSD (perimembranous inlet). Case 2 was a female, aged 15 months, who weighed 9 kg and was identified as No. 513389. The preoperative TTE showed VSD (membranous aneurysm type), with a base width of 7 mm and a top width of 3 mm.
Discussion
Following the clinical use of the Amplatzer muscular VSD occluder and the successful application of the Amplatzer VSD occluder for the treatment of perimembranous VSD by Hijazi et al. (1,2), Xing et al. (3) used a self-designed delivery system in 11 non-CPB closure cases with perimembranous VSD in 2007 in China. In recent years, non-CPB minimally invasive small incision transthoracic closure of VSD has been rapidly promoted and used in China due to its obvious advantages (4,5). Compared to conventional CPB surgical repair and catheter interventional closure, non-CPB minimally invasive small incision transthoracic closure of VSD offers several advantages: (I) CPB is unnecessary, and its related complications can be avoided; (II) under the guidance of TTE, the whole process of closure and its effect can be monitored, which avoids the damage of contrast agents and X-ray for both the patients and surgeons; (III) it is minimally invasive and rapid. The conventional transthoracic incision was 2–3 cm and the time of the whole conventional surgery was 40–50 min, compared to the shortest operative time in this study of 35 min; (IV) it is safe. If the therapeutic effect of closure cannot achieve the operational goal or if there is any abnormal situation, the patients can be switched to conventional CPB surgery at any time; (V) the interference of the occluder on the adjacent valves and outflow tract can be monitored in a timely fashion; (VI) the risk of intimal injury by interventional catheterization on the large vessel can be avoided; (VII) the age and weight restrictions for pediatric patients can be markedly relaxed (the lowest weight was 5.5 kg in this study).
In this study, the results showed that among 340 patients who underwent transthoracic closure of VSD, 26 (7.65%) patients needed to be switched to CPB intracardiac repair under direct visualization. During the first 1.5 years of the study, the surgeons learned and mastered the transthoracic closure, which could be regarded as a learning period. There are three groups of surgeons at our center, and the average learning period was approximately 60 cases. Among the 186 patients during the early period, 20 (10.75%) cases needed to be switched to CPB intracardiac repair under direct visualization, which was slightly higher than in some previous studies (4,5). During the subsequent 1.5 years, the surgeons had mastered the closure of VSD, and 6 out of 154 (3.90%) cases needed to be switched to CPB intracardiac repair under direct visualization. The success rate of the transthoracic closure was significantly improved, which was comparable with that of transcatheter medical closure of VSD (97.55%) (6) (Tables 2-4).
The conventional classifications of VSD are typically infundibular defects (subarterial and intracristal types), perimembranous defects (perimembranous, subseptal valve and membranous aneurysm types) and muscular defects. In this study, among the 186 patients who underwent closure of VSD during the early period, 20 cases were switched to CPB intracardiac surgery under direct visualization, including 11 cases of perimembranous type, 8 cases of membranous aneurysm type and 1 case of intracristal type. Among the 154 patients who underwent closure of VSD during the late period, 6 cases were switched to CPB intracardiac surgery, including 2 cases of perimembranous type, 3 cases of membranous aneurysm type and 1 case of intracristal type (Tables 4,5).
Regarding the specific causes for patients switching to CPB surgery due to failure of closure, among the 186 patients during the early period, the causes were failure of the guide wires to pass through the VSD (3.76%), significant residual shunts after releasing the occluder (2.69%), significant shifting or shedding after releasing the occluder (2.15%), significant interference on the movement of adjacent valves after releasing the occluder (1.61%) and severe complications (bleeding at the left atrial posterior wall) (0.54%). Among the 154 cases during the late period, the causes were significant residual shunts after releasing the occluder (1.95%), significant interference on the movement of adjacent valves after releasing the occluder (1.3%), and failure of the guide wires to pass through the VSD (0.65%).
During the early period, the reason why the guide wires could pass through the VSD was mainly associated with the proficiency of surgeons and the size of VSD, especially for the size of the right ventricular side of the perimembranous and membranous aneurysm VSD. It has been shown that residual shunts were the most common complication of failure of transthoracic closure, while the conventional view was that patients could be followed-up when the shunt beam was less than 1 mm and the speed was less than 2.5 m/s. There were also data showing that 50% of the transcatheter closure of VSD would develop small residual shunt post operation, which was 19% after 24 hours and only 4% after 6 months. In addition, neither the transcatheter closure nor surgical repair under direct visualization for VSD could completely prevent residual shunts (7). Therefore, it is necessary to establish a relative standard for the acceptable level of residual shunts post transthoracic closure.
Regarding the interference on the movement of adjacent valves after releasing the VSD occluder, our data showed that it was mainly the effect of the closure umbrella on the right coronary aortic valve and tricuspid valve, leading to unacceptable aortic regurgitation and tricuspid regurgitation (Case 1, Figures 1,2). Currently, there are no specific unified evaluation criteria for the regurgitation. The common causes for patients switching to open surgery under direct visualization due to failure of the transthoracic closure of membranous aneurysm VSD were: (I) in the preoperative TTE and intraoperative TEE examinations, several openings on the right ventricular side of membranous aneurysm were missing, as the findings of intraoperative CPB exploration in Case 2 (Figures 3,4); (II) the size of left ventricular side was underestimated, which was the main cause (especially for subseptal valve type of pseudo membranous aneurysm).
We believe that the main causes for patients switching to CPB surgery under direct visualization due to failure of transthoracic closure of VSD were different in different periods. The main causes at the early period were: (I) the guide wires could not pass through the VSD due to the skill proficiency of surgeons and size of VSD; (II) after releasing occluder, there were significant residual shunts; (III) after releasing, occluder had significantly shifting and shedding; (IV) after releasing, occluder significant interference on the movement of adjacent valves and aortic regurgitation. While the main causes at the late period were as follows: (I) after releasing occluder, there were significant residual shunts; (II) after releasing occluder, there were significant interference on the movement of adjacent valves and aortic regurgitation and aortic regurgitation; (III) the guide wires could not pass through the VSD. There was still no unified and reliable standard for the acceptable level of residual shunts and effect on valves after occluder releasing. It is required to conduct a multiple-center study with large-sample mid- and long-term follow-up to address this issue.
In conclusion, our study revealed that minimally invasive transthoracic occlusion was associated with a shorter duration of surgery, shorter ICU and hospital stay, fewer transfusions and a decreased incidence of post-operative arrhythmia. The type of VSD was the important protective factor and the size of the occluder increased the risk of conversion to open surgery. Residual shunting and aortic regurgitation were the main reason. A failure to establish the occluder conveying rail during the operation also was a reason, severe arrhythmia was another main reason for conversion to open surgery. Finally, tricuspid regurgitation was also cited as a reason for the high incidence of conversion to open surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Informed consent was obtained from each patient or the legal guardian before either procedure. The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University (Number of approval: GYFYY-2013-17).
References
- Tofeig M, Patel RG, Walsh KP. Transcatheter closure of a mid-muscular ventricular septal defect with an amplatzer VSD occluder device. Heart 1999;81:438-40. [Crossref] [PubMed]
- Hijazi ZM, Hakim F, Haweleh AA, et al. Catheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: initial clinical experience. Catheter Cardiovasc Interv 2002;56:508-15. [Crossref] [PubMed]
- Xing QS, Zhuang ZY, Pan SL, et al. Minimally invasive device closure of perimembranous ventricular septal defect with a new delivery system. Chin J Exp Surg 2007;24:1135-6.
- Xing QS, Pan SL, Wu Q. Minimally invasive perventricular VSD closure without cardiopulmonary bypass mid-term results from multi-centers. Chin J Thorac Cardiovasc Surg 2011;27:259-63.
- Wu Q, Gao L, Yang Y, et al. Echo-cardiography-guided occlusion of ventricular septal defect via small chest incision. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2012;37:699-705. [PubMed]
- Han Y, Tian J, Liu Q. Meta analysis of ventricular septal defect treated by transcatheter closure and surgical repair in mainland China. Chin J Evid Based Pediatr 2008;3:15-20.
- Carminati M, Butera G, Chessa M, et al. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardiol 2005;96:52L-58L. [Crossref] [PubMed]


