Management strategy of solitary pulmonary nodules
Lung cancer is currently the leading cause of cancer deaths worldwide (1). Clinically, most patients are diagnosed at an advanced stage, with only about 15% have the opportunity of surgical resection. Early detection followed by surgical resection of stage I lung cancer may lead to a 5-year survival rate of 54-73%, while those with stage IV diseases have a 5-year survival rate of only 2% (2,3). With the established role of low-dose helical computed tomography (CT) screening for lung cancer (4-6), and the wide application of high-resolution CT, solitary pulmonary nodules (SPNs) are increasingly detected (7). Accurate assessment, proper treatment and timely surgical resection of malignant pulmonary nodules will be highly beneficial to the survival of patients with lung cancer. By reviewing the latest literature, combined with our experience in the clinical management of SPNs, we summarized the relevant clinical problems and treatment strategies in this review.
Definition of pulmonary nodules
Currently, an accepted definition of SPNs is a single, well-circumscribed, radiographic opacity less than or equal to 30 mm in diameter that is completely surrounded by aerated lung and is not associated with atelectasis, hilar enlargement, or pleural effusion (8,9). SPNs can be caused by a variety of factors, including malignant diseases such as bronchogenic carcinoma, carcinoid tumors, lymphoma and single lung metastases from other tumors, or a range of benign lesions such as non-specific granuloma, specific granulomatous infections and hamartoma (10).
Pulmonary nodules should be characterized on the basis of number, size, and density. In recent years, an important type of pulmonary nodules has gradually increased, namely the sub-centimeter nodules, which refer to those in a diameter less than or equal to 8 mm (11). Studies have shown that sub-centimeter lung nodules have an overall low degree of malignancy (12). With high-resolution CT, lung nodules can be categorized in a more accurate and detailed way. Ground-glass opacity (GGO) is a special type of pulmonary nodules. GGO is a sign of slightly increased density on the high-resolution CT, in which the bronchial and vascular textures are still visible (13). This sign is a characteristic instead of specific imaging finding, which can be found in multiple lesions in the lung (14). Based on the presence of solid tissue component on high-resolution CT, GGO can be classified into three types: pure GGO (pGGO), mixed GGO (mGGO) and solid nodules (15).
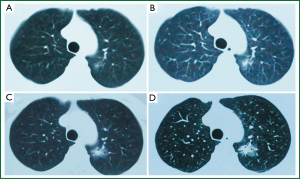
In clinical settings, we often encounter patients with GGO, and delayed diagnosis is common due to suboptimal follow-up visits. A 47-year-old female patient without history of smoking was diagnosed with right tuberculous pleurisy in our clinical center in 2002. Following anti-tuberculosis treatment, the patient came to our tuberculosis clinic for follow-up visits every 2-3 years. In April 2006, the chest CT scan indicated GGO in the left upper lung, in the size of about 8 mm (Figure 1A). At the follow-up visit in January 2008, the chest CT scan found GGO in the same location in a slightly increased size of about 10 mm, with central consolidation (Figure 1B). In June 2011, another follow-up CT showed significant enlargement of the GGO lesion in the left upper lung to about 16 mm (Figure 1C). However, in October 2012, the chest CT scan lesions showed significant enlargement to about 24 mm, with increased central consolidation and burr changes around the lesion (Figure 1D). A positron emission tomography (PET)/CT scan indicated a SUV of 1.7 of the left upper lung lesion. Surgery was performed to remove the lesion directly, and postoperative pathology indicated adenocarcinoma at stage IA.
Assessment of the probability of malignancy for pulmonary nodules
The probability of malignancy varies depending on the size of pulmonary nodules. Lesions larger than 30 mm in diameter are defined as masses instead of nodules. Resent study results have shown that masses are more likely suggestive of malignancy (15). In many lung cancer screening trials, the probability of malignancy is 0-1% among pulmonary nodules smaller than 5 mm in diameter, 33-64% in those from 11 to 20 mm in diameter, and up to 64-82% in nodules larger than 20 mm (16). The boundary of nodules is also helpful in evaluating the malignancy. In general, irregular, lobulated or burr-like boundaries are more likely to be malignant, compared with a smooth margin (16). Compared with solid nodules, ground-glass opacities or semi-solid pulmonary nodules are more likely malignant (17).
Evaluation and determination the probability of malignancy for pulmonary nodules is essential to the subsequent management and treatment. First of all, an assessment of the probability should be conducted based on a patient’s clinical risk factors and characteristics of the pulmonary nodules on CT images (10). The clinical evaluation includes a review of medical history and examination of symptoms. High probability of malignancy is correlated with such clinical risk factors as the nodule size, age, history of cancer, smoking history, history of chronic obstructive pulmonary disease, and history of asbestos exposure. At present, the most accepted criteria is the evaluation criteria for probability of malignant in pulmonary nodules put forward by Ost et al. see Table 1 (8,12,14).
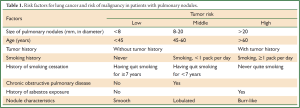
Full table
Management of solitary pulmonary nodules (SPNs)
Basic management for patients with pulmonary nodules includes three steps: (I) continuous CT scans for close follow-up observation; (II) further diagnostic tests (imaging, biopsy, or a combination of both); and (III) surgical resection. Obviously, if the probability of malignancy is 0, careful follow-up observation will be the optimal choice. Conversely, if the probability is close to 1, surgical resection following an appropriate staging will be the most appropriate option. For those with a malignant probability between 0 and 1, further examination is the best choice (16). These three steps will be elaborated as below, with emphasis on the timing, supporting signs and limitations, as well as the uncertainty.
Careful follow-up observation
This mainly refers to the continuous monitoring of CT scans, usually applied for patients with a relatively low probability of malignancy before the test (<5-10%) (10). Many lung cancer screening trail results show that careful follow-up observation is an optimal treatment strategy as screening is often used among those with pulmonary nodules that have a relatively low probability of malignancy (18,19). This strategy is mainly limited by the uncertainty in which a risk of delayed diagnosis is possible, especially when metastases are likely to occur during the observation period, which could have been prevented by early surgical resection. Although the optimal imaging technique is yet to be identified, Fleischner Society has recommended a consensus on the follow-up timing for lung nodules, mainly based on nodule size and presence or absence of risk factors for lung cancer (20,21). The details are shown in Table 2 (22).

Most malignant lesions have a growth doubling time of 20-300 days. Therefore, clinicians tend to accept that stable imaging results for two years are indicative of benign lesions (23,24). Some studies have shown that pGGO, semi-solid lesions and solid lesions have an average doubling time of 813, 457, and 149 days, respectively (25). Thus, some investigators suggest that once pGGO nodules are found, otherwise healthy patients should receive follow-up imaging visits for more than two years. Despite the above shortcomings, stable imaging findings for two years are still considered an important basis for differentiating between benign and malignant nodules. For certain patients with GGO or semi-solid nodules, the follow-up period can be properly extended. Growth acceleration or generation of solid components confirmed by continuous CT scans will warrant further histological diagnosis, often via CT-guided needle biopsy or surgery (8).
Diagnostic testing
When the probability of malignant nodules is at the moderate level of around 10-60%, further diagnostic testing is the recommended strategy. These may include PET/CT, CT-guided needle biopsy and bronchoscopy.
PET
Studies have shown that the sensitivity and specificity of PET for the diagnosis of malignant lesions can reach 87% and 83%, respectively (20). When the pre-test probability of malignancy is low and PET results are negative, careful follow-up observation can be considered. However, PET also has its shortcomings. Firstly, PET is not sensitive for nodules smaller than 8-10 mm in diameter (26). For patients with in situ adenocarcinoma, carcinoids and mucinous adenocarcinoma, PET may provide a false negative result, and false positive findings may occur in patients who have inflammatory reactions (sarcoidosis or rheumatoid nodules) or in a status of infection (fungal or mycobacterial infections).
CT-guided fine-needle aspiration biopsy (FNAB)
FNAB is a common method for lung tissue biopsies in clinical settings, particularly for SPNs located close to the chest wall. The diagnostic accuracy mainly depends on an operator’s positioning and puncturing skills, in addition to the pathology technical level that may have a certain impact on the results.
Fiber optic bronchoscopy (FOB)
FOB-based pathological techniques for the diagnosis of SPNs include bronchial brush cytology (BB), bronchial alveolar lavage (BAL) and transbronchial lung biopsy (TBLB). The development of endobronchial ultrasound, ultrathin bronchoscopy and electromagnetic navigation has improved the sensitivity of TBLB.
Surgery
In the case of a high probability of malignant pulmonary nodules (>60-70%), video-assisted thoracoscopic surgery (VATS) is the recommended strategy, for it satisfies the needs of both diagnosis and further treatment. With a benign result from intraoperative frozen pathology, only wedge resection will be needed. For malignant pathological findings, surgical resection should be selected in combination with systematic lymph node dissection.
Clinical pathway for management of pulmonary nodules
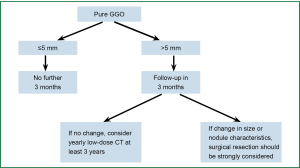
The treatment strategy for pulmonary nodules should be developed taking into account the probability of malignancy, risk of surgery, difficulty of diagnostic testing and individual preference of patients. The decision-making process should begin from a review of the medical history and physical examination, for the purpose of assessing the tumor probability and risk of surgery. We have developed a new strategy flowchart for the management of SNPs based on the specific strategies in 2013 ACCP guidelines for lung cancer diagnosis and treatment (27), in combination with the clinical experience presented by Ost (10). The strategy for solid pulmonary nodules is depicted in Figure 2. In view of the slow growth rate of GGO nodules, specific treatment is required for such GGO or semi-solid lesions. We recommend follow-up examinations shortly after a patient visit, followed by diagnostic puncture or surgical resection to yield a pathological diagnosis as soon as possible. The process is detailed in Figure 3.
To sum up, the diagnosis and treatment of pulmonary nodules should start from assessing the probability of malignancy, and in turn evaluating the pros and cons of surgery as well as the consequences of treatment, while taking into account a patient’s physical condition, complications, and personal preference. Surgery is preferred for patients with a high probability of malignancy. For those with a moderate malignant probability, CT-guided needle biopsy or PET scans will be the best choice.
Acknowledgements
The study was supported by a grant from “Twelve-Five Plan’’, the Major Program of Nanjing Medical Science and Technique Development Foundation (Molecular Mechanism Study on Metastasis and Clinical Efficacy Prediction of Non-small Cell Lung Cancer) and Third Level Training Program of Young Talent Project of Nanjing Health, Nanjing Medical Science and Technology Development Project (QRX11226), and Young Professionals Foundation of Nanjing Chest Hospital.
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Colt HG, Murgu SD, Korst RJ, et al. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e437S-54S.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Marshall HM, Bowman RV, Yang IA, et al. Screening for lung cancer with low-dose computed tomography: a review of current status. J Thorac Dis 2013;5:S524-S539. [PubMed]
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 1: radiologic characteristics and imaging modalities. Chest 2013;143:825-39. [PubMed]
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [PubMed]
- Wu YL, Jiang GL, Liao ML, et al. Management of solitary pulmonary nodules. Evid Based Med 2009;9:4.
- Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 2012;185:363-72. [PubMed]
- Xu DM, van der Zaag-Loonen HJ, Oudkerk M, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology 2009;250:264-72. [PubMed]
- Slattery MM, Foley C, Kenny D, et al. Long-term follow-up of non-calcified pulmonary nodules (<10 mm) identified during low-dose CT screening for lung cancer. Eur Radiol 2012;22:1923-8. [PubMed]
- Austin JH, Müller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327-31. [PubMed]
- Song Y, Zhan P. Management and differential diagnosis of Ground-Glass Opacity pulmonary lesions. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:808-9. [PubMed]
- Ost D, Fein A. Evaluation and management of the solitary pulmonary nodule. Am J Respir Crit Care Med 2000;162:782-7. [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Goo JM, Park CM, Lee HJ. Ground-glass nodules on chest CT as imaging biomarkers in the management of lung adenocarcinoma. AJR Am J Roentgenol 2011;196:533-43. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259-65. [PubMed]
- Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000;215 Suppl:607-9. [PubMed]
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest 2007;132:108S-30S.
- Takashima S, Sone S, Li F, et al. Small solitary pulmonary nodules (< or =1 cm) detected at population-based CT screening for lung cancer: reliable high-resolution CT features of benign lesions. AJR Am J Roentgenol 2003;180:955-64. [PubMed]
- Ko JP, Berman EJ, Kaur M, et al. Pulmonary nodules: growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology 2012;262:662-71. [PubMed]
- Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. [PubMed]
- Herder GJ, Golding RP, Hoekstra OS, et al. The performance of 18F-fluorodeoxyglucose positron emission tomography in small solitary pulmonary nodules. Eur J Nucl Med Mol Imaging 2004;31:1231-6. [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 2: pretest probability and algorithm. Chest 2013;143:840-6. [PubMed]