Effect of comorbidities on long-term outcomes after thoracoscopic surgery for stage I non-small cell lung cancer patients with chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a recognized risk factor for lung cancer, one of the most common cancers worldwide. The risk of lung cancer in patients with COPD is approximately five-fold greater than that in smokers without COPD (1). The close relation of these two diseases means that clinicians often encounter patients with coexisting COPD and lung cancer. Several studies have suggested that patients with comorbid lung cancer and COPD have higher risk of postoperative complications, and that their prognosis was worse than in patients with lung cancer but without COPD (2,3). Sekine et al., for example, reported that patients with COPD had poorer long-term survival because tumor recurrence was more common (4).
Improvements in operative and perioperative management, mean that patients with poor pulmonary function and severe comorbidities can now undergo procedures that were previously contraindicated. Nevertheless, Battafarano et al. reported that comorbidity remained an independent prognostic factor in patients with resected stage I non-small cell lung cancer (NSCLC) (5). In these patients, COPD is often present with other severe comorbidities, that may adversely influence long-term survival, but there have been no reports of differing roles of COPD and other comorbidities in patients with resected lung cancer.
Thoracoscopic surgery (TS) for lung cancer has been increasingly used over recent years. Advantages include its minimal invasiveness, reduced postoperative pain and complications, and shortened hospital stays. In research by Jeon et al., lobectomy by video-assisted thoracic surgery (VATS) was associated with a lower incidence of pulmonary complications compared with lobectomy by thoracotomy in patients with stage I NSCLC and COPD (6). TS may, therefore, improve safety and postoperative outcomes in these patients. However, it can be difficult to secure a safe operating field in TS for patients with COPD because lung collapse is poor, and the poor pulmonary parenchyma increases the risk of secondary injury. Due to these issues, TS is still controversial for patients with COPD, and the outcomes in patients with comorbid lung cancer have not been well studied (6-8).
At our hospital, we have performed lung resection by TS for all patients with stage I lung cancer, regardless of the diagnosis of COPD, since 2006. In this study, we had three aims. First, we aimed to assess the impact of the COPD severity on the long-term survival of patients with NSCLC after lung resection by TS. Second, we aimed to assess whether other comorbidities influenced long-term survival in these patients. Third, we aimed to assess the safety and effectiveness of lung resection by TS for patients with NSCLC and COPD.
Methods
Patients
This retrospective study was approved by the Ethics Committee of Jikei University School of Medicine, the Number is 29-069 (8685), which waived the requirement for informed consent from patients.
We surveyed the thoracic surgery database in our hospital for patients who underwent lung resection for c-stage I NSCLC between January 2006 and December 2014 in our hospital. We excluded patients with adenocarcinoma in situ or a history of surgery for lung cancer.
Data collection and definitions
All patients underwent preoperative evaluation. This included a detailed medical history, physical examination, blood and urine examinations, spirometry, and electrocardiography. Smoking status was examined based on patient questionnaires. Preoperative tumor staging was by chest X-ray, contrast-enhanced chest and abdominal computed tomography, brain magnetic resonance imaging, and bone scintigraphy. Staging and pathology were based on the seventh revision of the TNM staging criteria (9).
COPD was defined as a forced expiratory volume in 1 second (FEV1) divided by a forced vital capacity (FVC) that was <0.7 in the presence of a smoking history. We used the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric grades to classify the severity of airflow limitation, as follows (10): GOLD 1 (mild), FEV1 ≥80% predicted; GOLD 2 (moderate), FEV1 ≥50% but <80% predicted; GOLD 3 (severe), FEV1 ≥30% but <50% predicted; and GOLD 4 (very severe), FEV1 <30% predicted. Patients diagnosed with COPD were generally treated with inhaled bronchodilators. Regardless of the presence or absence of COPD, respiratory therapy was used before and after surgery.
We included information on comorbidity for each patient before lung cancer was diagnosed. Comorbidity was classified according to the Charlson comorbidity index (CCI) (11). In the CCI, diseases are grouped into 19 categories, each of being assigned a score of 0–6 depending on the assessed severity. The CCI is then given as the sum across these categories. Patients were grouped by CCI into those with scores of 0, 1–2, and ≥3).
Concerning surgery, anatomical lobectomy with mediastinal lymph node dissection was the preferred surgery. However, we selected sublobar resection for patients in whom the postoperative FEV1 was predicted to be less than 800 mL and for patients who had severe comorbidities and were not expected to tolerate lobectomy.
Postoperative complications of interest included prolonged air leak, bronchial stump fistula, empyema, pneumoderma, pleural effusion, chylothorax, pneumonia, recurrent nerve paralysis, arrhythmia, and postoperative home oxygen therapy (HOT) use. Prolonged air leak was defined as a persistent air leak for >7 days, reinsertion of a chest tube due to pneumothorax, or reoperation because of a diagnosed prolonged air leak.
Follow-up and survival outcomes
The postoperative administration of tegafur-uracil was indicated for selected patients with pathological stage IB and platinum-based chemotherapy for patients with pathological stage IIA or more. After discharge, follow-up visits were performed every 6 months for the first 5 years and annually thereafter. Physical examination, serum tumor makers, and contrast-enhanced chest computed tomography scans were performed at each visit.
We measured the overall survival (OS) and disease-specific survival (DSS) for each patient group. OS was measured from the date of surgery to the date of death from any cause or date the patients was last known to be alive. DSS was measured from the date of surgery to the date of death from the lung cancer.
Statistical analysis
We used JMP 12 (SAS Institute Inc., Cary, NC, USA) to perform the statistical analyses. Date are reported as median (range), and patient characteristics were compared between the three groups using the Pearson χ2 test for categorical variables or the Wilcoxon test for continuous variables. The Kaplan-Meier method was used to plot data curves, and log-rank tests were used to analyze OS and DSS. To evaluate the significance of factors related to OS and DSS, univariate and multivariate analyses were performed by the Cox proportional hazards method. P<0.05 were considered statistically significant.
Results
Of the 677 patients who underwent lung resection for cancer during the study period, 458 patients had c-stage I NSCLC. We excluded a further 35 patients with adenocarcinoma in situ, 18 patients with a history of surgery for lung cancer, and 1 patient who underwent open thoracotomy for simultaneous resection of esophageal and lung cancer. No patients received preoperative chemotherapy or radiation therapy. Thus, 404 eligible patients with c-stage I NSCLC were included, and their characteristics are summarized in Table 1.
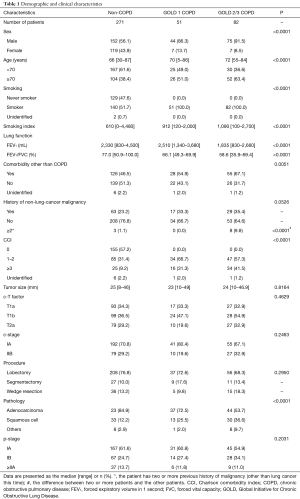
Full table
The median follow-up time among survivors was 51.0 months, and all cases underwent lung resection by TS. Among the 404 patients, 133 were diagnosed with COPD and 271 were not diagnosed with COPD (non-COPD). Of those with COPD, 51, 79, and 3 were classified as GOLD 1, GOLD 2, and GOLD 3, respectively. Male sex, older age, positive smoking history, and squamous cell carcinoma were more frequent in the COPD group than in the non-COPD group. However, more patients had comorbidities other than COPD in the GOLD 2/3 group (P=0.0051). There were no significant differences in the number of patients with non-lung-cancer malignancy between the three groups (P=0.0526), but significantly more patients in the GOLD 2/3 COPD group had at least two non-lung-cancer malignancies (P<0.0001). The CCIs were 0, 1–2, and ≥3, respectively, in 57.2%, 31.4%, and 9.2% of the non-COPD group, in 0%, 66.7%, and 31.3% of the GOLD 1 group, and in 0%, 57.3%, and 41.5% of the GOLD 2/3 group (P<0.0001). There were no significant differences in clinical stages, the pathological stages, or surgical procedures.
The surgical outcomes are summarized in Table 2. The operative time and frequency of conversion to thoracotomy did not differ among the three groups. Postoperative complications were significantly more frequent in the GOLD 1 (21.5%) and GOLD 2/3 (26.8%) groups than in the non-COPD group (12.1%) (P=0.0040). In particular, prolong air leak was more frequent in the GOLD 2/3 group (17.1%) than the non-COPD (5.9%) or GOLD 1 groups (7.8%). No patients died within 30 days after surgery.
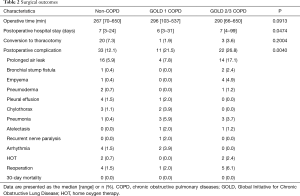
Full table
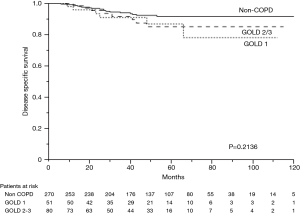
The 5-year OS rates were 86.0% in the non-COPD group, 80.2% in the GOLD 1 group, and 71.1% in the GOLD 2/3 group (P=0.0221, Figure 1); the corresponding 5-year DSS rates were 91.7%, 86.9%, and 85.1% (P=0.2136, Figure 2). Univariate analysis indicated that sex, smoking status, pathology, COPD severity, CCI, and pathological stage were associated with OS (Table 3). Multivariate analysis confirmed that only the CCI and pathological stage were associated with OS; interestingly, COPD severity was not associated with OS (Table 4).
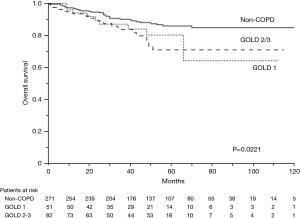
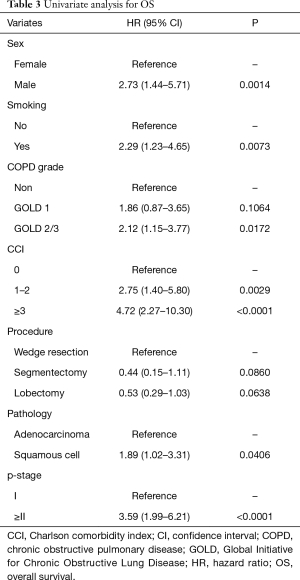
Full table
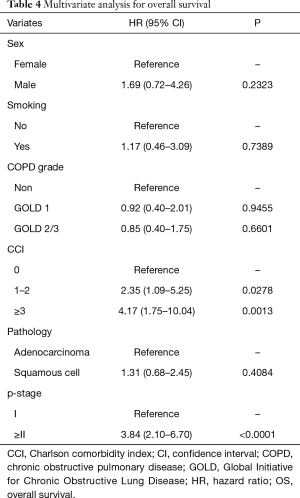
Full table
Discussion
In this study, patients with c-stage I NSCLC and COPD had a poorer OS than patients without COPD, but it was the CCI and not the COPD severity that was an independent prognostic factor for OS. Comorbidities adversely affected the long-term survival of patients with NSCLC and COPD after TS, and the same effect can be oncologically expected regardless of the COPD severity. In this study, lung resection by TS for NSCLC in patients with COPD was associated with good long-term survival and acceptable postoperative complication rates.
Several studies have indicated that patients with COPD are at increased risk of postoperative complications after surgery, for lung cancer and that their prognosis is worse than that of patients without COPD (2,3). Sekine et al. reported that patients with stage IA lung cancer and COPD had poorer long-term survival due to higher recurrence rates than patients without COPD, and that COPD was an independent prognostic factor (4). They concluded that COPD may be a strong promoting factor for lung cancer by accelerating postoperative recurrence and metastasis. Two other studies reported that higher COPD grades were associated with higher postoperative pulmonary complications and poorer long-term survival rates (3,12).
Patients with COPD often have comorbidities such as other malignancies, cardiovascular disease, and diabetes. In our study, there were more of these other comorbidities, and the CCI was higher in patients with COPD than in patients without COPD. In previous studies examining prognosis in patients with lung cancer and COPD, other comorbidities were not investigated, though several studies reported that comorbidities affected prognosis after NSCLC resection (5,13). Therefore, we did not know the extents to which COPD and other comorbidities adversely affected the prognosis of patients with COPD. Due to this, we examined whether the COPD severity (by the GOLD criteria) and the level of comorbidity (by the CCI) influenced long-term survival among patients with NSCLC. Our research showed that patients with COPD had a poorer OS than patients without COPD, but that there was no significant difference in the DSS. Moreover, it was the CCI, not the COPD severity that turned out to be an independent prognostic factor. This suggests that CCI adversely affects long-term survival to greater degree than the COPD severity in patients with stage I lung cancer.
The importance of comorbidities on cancer survival was raised by Feinstein et al. in 1985 (14). Several studies have since investigated the effect of comorbidity on survival after NSCLC resection, and using different indices to measure comorbidity, an independent prognostic effect has been shown (5,15). The CCI, which was developed in 1987, is one method of classifying the prognostic effect of comorbidity (11). Since its introduction, several studies have reported that the CCI is a prognostic factor for patients after NSCLC resection (16,17). We used the CCI to classify comorbidities because it is currently the most widely used index in clinical and research practice. Our research was consistent with previous research suggesting that CCI is an independent prognostic factor.
Since the early 1990s, TS has rapidly developed and been widely applied worldwide. Paul et al. reported that patients undergoing lobectomy by TS had similar long-term survival outcomes, but shorter hospital stays, and lower in-hospital mortality rates than patients undergoing lobectomy by thoracotomy (18). Recently, reports showing the effectiveness of TS are increasing (19-23). Endoh et al., for example, reported that pulmonary function was better after pulmonary resection in patients who underwent VATS than in patients who underwent open thoracotomy (24). Moreover, there are several reports of the benefits of TS for patients with poor pulmonary function (6,25). Although TS may benefit long-term smokers, patients with poor pulmonary function, and high-risk patients with multiple comorbidities, it remains controversial for patients with COPD. Despite this, we have used TS aggressively to treat lung cancer patients regardless of COPD diagnosis, since 2006. We performed lung resection by TS in all 404 eligible participants for this study. Only one patient with c-stage I lung cancer underwent open surgery, and that was because they required simultaneous resection of esophageal cancer. TS for patients with NSCLC and COPD was associated with good long-term survival and acceptable postoperative complications in our hospital. After TS for patients with NSCLC, there was no significant difference observed in DSS between any COPD severity group. Similar effects were also oncologically obtained for patients with GOLD 2/3 COPD.
Several studies have reported that patients with COPD develop postoperative complications more frequently than patients without COPD, with prolonged air leak being most common. The incidence of air leak greatly affects the postoperative complication rate and length of hospitalization. Magdeleinat et al. reported that complications after thoracotomy were observed in 70%, and that prolonged air leak occurred in 17.0%, of patients with severe COPD (26). By contrast, Wang et al. reported that postoperative complications were observed in 36.1%, and that prolonged air leak occurred were 26.2%, of patients with lung cancer and severe COPD who underwent VATS lobectomy, which was significantly lower compared with previous studies of thoracotomy (7). According to the report by Cui et al., patients who underwent complete VATS had significantly lower morbidity than those who underwent VATS combining both direct and televisual monitoring (8). In our study, 33 patients (24.8%) had postoperative complications in the COPD group, which was significantly higher than that in the non-COPD group. Prolonged air leak was the most frequent postoperative complication in all three groups, but its frequency was higher in the GOLD 2/3 group than in the non-COPD group. The frequency of prolonged air leak was comparable to that reported in earlier studies, but the overall frequency of all postoperative complications was lower than in past studies. We experienced recurrent nerve paralysis in one out of 404 cases; however, it was transient, and the patient was discharged on the 8th day after surgery without developing other complications, such as pneumonia.
Cardiovascular complications are among the most serious postoperative complications. Pompili et al. reported that postoperative cardiopulmonary complications were more frequent in lung cancer patients with COPD than in those without COPD (27). Magdeleinat et al. reported that the cardiovascular complications occurred in 17.0% of patients with NSCLC and severe COPD (26), whereas Cui et al. reported a frequency of 10.5% (8). In our study, the frequencies of cardiovascular complications were lower than in past reports, with two patients (1.5%) in the COPD groups developing arrhythmias and four patients (1.5%) in the non-COPD group developing arrhythmias. The minimal invasiveness of TS may have reduced the overall postoperative complication rates. It is possible that TS is better able to preserve sufficient postoperative lung function which may lower the postoperative complication rate and improve long-term survival.
Though several theories exist, the precise biological mechanisms through which COPD influence the prognosis of NSCLC are unclear. Sekine et al. have suggested that mutation, abnormal expression, or suppression of a yet to be identified gene may accelerate lung cancer recurrence and metastasis in patients with COPD (4). In our study, patients with stage I NSCLC and COPD had a poorer OS than patients without COPD, but there was no significant difference in the DSS. Moreover, CCI rather than COPD was an independent prognostic factor. Patients with COPD also had more comorbidities, and those comorbidities adversely affected OS. It is important to remember that smoking may be causative in several comorbidities, including diabetes, cardiovascular diseases and other malignancies, and not merely COPD. These other diseases themselves cause systemic inflammation and interact with each other, potentially adversely affecting long-term survival in ways that have not been adequately considered. Despite this possibility, smoking history was not shown to be an independent prognostic factor in this study, indicating that there may be inter-individual differences in vulnerability to smoking.
There are several limitations to this study. First, this was a retrospective study with the inherent limitations of such design. Second, we included a small sample size from a single center. Third, COPD was only diagnosed on the basis of smoking status and airflow limitations, whereas it is known that COPD should be defined by diffusion capacity, radiological findings, and clinical symptoms, and not simply functional limitation. Fourth, the CCI used in this study was based on the original method presented in 1987, but a recent publication has proposed that some of the conditions in the score need re-weighting to reflect the reductions in mortality due to treatment advances over recent decades (28). Based on these limitations, it is possible that confounding variables may have been missed, some cases may have been incorrectly included or excluded, and that comorbidities may have been incorrectly weighted. Therefore, our results should be verified in a large prospective study designed to mitigate against these limitations.
Conclusions
In conclusion, patients with c-stage I NSCLC and COPD had a poorer OS than patients without COPD. However, it was the CCI rather than the COPD severity that was the main independent prognostic factor for OS. Our data also showed that the presence of comorbidities adversely affected long-term survival in patients with NSCLC and COPD after TS, and the same effect can be oncologically expected regardless of the COPD severity. Lung resection by TS was associated with good long-term survival and acceptable postoperative complication rates for these patients. We therefore conclude that TS is safe and effective for patients with c-stage I NSCLC and comorbid COPD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Jikei University School of Medicine [9-069 (8685)] and informed consent from patients was waived.
References
- Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. [Crossref] [PubMed]
- Zhai R, Yu X, Shafer A, et al. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest 2014;145:346-53. [Crossref] [PubMed]
- Sekine Y, Suzuki H, Yamada Y, et al. Severity of Chronic Obstructive Pulmonary Disease and Its Relationship to Lung Cancer Prognosis after Surgical Resection. Thorac Cardiovasc Surg 2013;61:124-30. [PubMed]
- Sekine Y, Yamada Y, Chiyo M, et al. Association of chronic obstructive pulmonary disease and tumor recurrence in patients with stage IA lung cancer after complete resection. Ann Thorac Surg 2007;84:946-50. [Crossref] [PubMed]
- Battafarano RJ, Piccirillo JF, Meyers BF, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2002;123:280-7. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy:a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- Wang W, Xu Z, Xiong X, et al. Video-assisted thoracoscopic lobectomy for non-small cell lung cancer in patients with severe chronic obstructive pulmonary disease. J Thorac Dis 2013;5:S253-9. [PubMed]
- Cui F, Liu J, Shao W, et al. Thoracoscopic minimally invasive surgery for non-small cell lung cancer in patients with chronic obstructive pulmonary disease. J Thorac Dis 2013;5:S260-6. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project:proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;152:S77-121. [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies:development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Yoshida Y, Kage H, Murakawa T, et al. Worse prognosis for stage IA lung cancer patients with smoking history and more severe chronic obstructive pulmonary disease. Ann Thorac Cardiovasc Surg 2015;21:194-200. [Crossref] [PubMed]
- Lüchtenborg M, Jakobsen E, Krasnik M, et al. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur J Cancer 2012;48:3386-95. [Crossref] [PubMed]
- Feinstein AR. On classifying cancers while treating patients. Arch Intern Med 1985;145:1789-91. [Crossref] [PubMed]
- Wang CY, Lin YS, Tzao C, et al. Comparison of Charlson comorbidity index and Kaplan-Feinstein index inpatients with stage I lung cancer after surgical resection. Eur J Cardiothorac Surg 2007;32:877-81. [Crossref] [PubMed]
- Moro-Sibilot D, Aubert A, Diab S, et al. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J 2005;26:480-6. [Crossref] [PubMed]
- Otake S, Ohtsuka T, Asakura K, et al. Impact of comorbidity index on morbidity and survival in non-small cell lung cancer. Asian Cardiovasc Thorac Ann 2016;24:30-3. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracosopic versus open lobectomy:propensity matched comparative analysis using SEER-Medicare datebase. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy:a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy:a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81. [Crossref] [PubMed]
- Berry MF, D'Amico TA, Onaitis MW, et al. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg 2014;98:197-202. [Crossref] [PubMed]
- Endoh H, Tanaka S, Yajima T, et al. Pulmonary function after pulmonary resection by posterior thoracotomy, anterior thoracotomy or video-assisted surgery. Eur J Cardiothorac Surg 2010;37:1209-14. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function:a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Magdeleinat P, Seguin A, Alfano M, et al. Early and long-tern results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg 2005;27:1099-105. [Crossref] [PubMed]
- Pompili C, Brunelli A, Refai M, et al. Dose chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010;37:525-30. [Crossref] [PubMed]
- Quan H, Li B, Couris CM, et al. Updating and validating the Chalrson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6countries. Am J Epidemiol 2011;173:676-82. [Crossref] [PubMed]


