Implantable cardioverter defibrillator therapy in grown-up patients with transposition of the great arteries—role of anti-tachycardia pacing
Introduction
Improvements in cardiac care of children with congenital heart defects have led to increasing numbers of long-term survivors reaching adulthood. Approximately 85% of babies born with heart defects can now be expected to reach adulthood (1). Adults with congenital heart disease are at increased risk of death compared to the general population (2,3). Among those with the highest risk for sudden cardiac death (SCD) are patients with dextro-transposition of the great arteries (dTGA) following Mustard/Senning procedure and patients with congenitally corrected transposition of the great arteries (ccTGA) (2,4-6). Therapy with an implantable cardioverter defibrillator (ICD) has been shown to prevent SCD in a wide range of patients at risk (7,8). Here we report our single center long-term experience with ICD therapy in patients with dTGA or ccTGA.
Methods
Patients und follow-up
All ICD carriers with surgically corrected dTGA and burdened right ventricle or with ccTGA who were in active follow-up at our institution in November 2013 were included. Past medical history and ICD follow-up data were obtained retrospectively from hospital records. All patients were then followed until December 31, 2015. Endpoint of follow-up was death, heart transplant or implantation of a ventricular assist device. Follow-up was also terminated for explantation of the ICD or for battery depletion without generator replacement.
Secondary prevention indication for ICD implantation was defined as a documented history of cardio-pulmonary resuscitation due to ventricular tachyarrhythmias. Primary prevention was defined as an ICD implantation based on the assessment of the risk of SCD.
The study conforms to the Declaration of Helsinki. The requirement of informed consent for this observational study was waived by the institutional committee on human research.
Episode and device data acquisition
Episode data were obtained from stored programmer data and printouts. Original intracardiac electrograms (IEGM) were analyzed. Multiple episodes in short succession were counted as a single episode.
Device parameters changed over time, reflecting physician-tailored programming in response to individual arrhythmia burden. Therefore, we report the first permanent device programming (defined as the setting active after the 3-month follow-up) of the first pulse generator. Where detection duration was given in seconds, as in Boston Scientific or former Ventritex devices, detection interval counter was calculated using the programmed detection rate. Detection interval counter cannot be changed in totally subcutaneous ICDs (S-ICD) and is therefore reported as per manufacturer settings, 18 out of 24 intervals, for these devices (9).
Statistics
Data are reported as mean ± standard deviation or median and interquartile range (IQR), depending on normality of distribution. Continuous variables were compared using the two-sided unpaired Student’s t-test. Homogeneity of variance was tested using Levene’s test with P<0.1 considered heterogeneous. The 95% confidence intervals (CI) are given where appropriate. Chi-square test and Fisher’s exact test were used for comparison of categorical variables. A P value <0.05 was considered statistically significant. Mean time to events was calculated using a Kaplan-Meier analysis. Drug effects on the cycle length were calculated using a univariate linear model. Estimated marginal means of the cycle length adjusted to a monotherapy of the individual drug are reported. All calculations were performed using SPSS 23 (SPSS Inc., Chicago, IL, USA).
Results
Patients
Fourteen ICD carriers with transposition of the great arteries or congenitally corrected transposition were included in the analysis. Median follow-up duration was 5.0 years (IQR, 2.2–15.1 years). Cumulative follow-up added up to 113.5 patient-years. Patient characteristics are shown in Table 1.
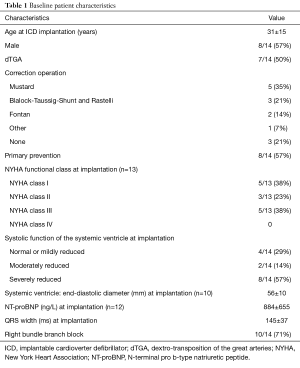
Full table
Of the 14 patients initially identified and followed-up, 3 reached an endpoint of follow-up between November 2013 and December 31, 2015. One patient died suddenly due to massive pulmonary embolism. Another patient received a ventricular assist device. The third patient declined generator exchange at the end of battery life. This patient had experienced multiple inappropriate shocks for atrial fibrillation before. There were no arrhythmic deaths during follow-up.
ICD, implantable cardioverter defibrillator; dTGA, dextro-transposition of the great arteries; NYHA, New York Heart Association; NT-proBNP, N-terminal pro b-type natriuretic peptide.
ICD device and programming data
Including pulse generator exchanges and lead revisions, 28 devices were implanted in 30 operations. Single chamber ICDs were implanted in 5 patients (36%), dual chamber ICDs in 6 patients (43%) and a CRT-D system in one patient (7%). Two patients (14%) received an S-ICD. Initial Device programming is shown in Table 2. In 11 patient 2 zones (VT and fast VT/VF) were programmed, while 3 devices (all implanted before the year 2000) were programmed to a single (VF-) zone.
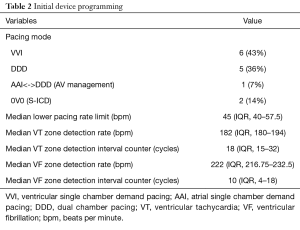
Full table
Ventricular tachyarrhythmias
Nine patients (64%) experienced ventricular tachyarrhythmias during follow-up which were treated by the ICD. Eight of these experienced ICD shocks, while in one patient all arrhythmias were successfully terminated by anti-tachycardia pacing (ATP). No significant difference in the occurrence of ventricular tachyarrhythmias was observed between patients with primary or secondary prevention indication (P=0.3, Fisher’s exact test) (Table 3). Mean time to first ventricular episode in a Kaplan-Meier analysis was 1966 days (95% CI, 718–3,215 days). Patients with ventricular tachyarrhythmias had a median of 1.0 (IQR, 0.46–7.1) episodes per year. The maximum arrhythmia burden was observed in a patient with 53 VT/VF episodes within 548 days (35.3 episodes per year).

Full table
A total of 177 ventricular episodes occurred during follow-up. At least one ATP was delivered in 128 episodes (72%) (Figure 1). ATP was successful in 102 episodes (80% of episodes treated with ATP). Twenty-two of the episodes with unsuccessful ATP were terminated by ICD shock. Six arrhythmias were not treated successfully by the ICD: 3 hemodynamically stable slow VTs decelerated below detection rate after the last ATP without termination. Two other slow VTs had a rate near the detection rate undulating at the VT cut-off rate, being detected as non-sustained arrhythmia without ICD therapy attempt. These arrhythmias were hemodynamically well tolerated by the patients. Another episode consisted of a VT occurring while the patient was hospitalized at the ICU. ATPs accelerated the VT into ventricular fibrillation which was defibrillated externally before an ICD shock could be delivered.
Cycle length was 310±72 ms, being available in 91% of 177 episodes. About 83% of ventricular episodes occurred while the patient was taking beta-blockers (other than sotalol), 14% on sotalol, 28% on amiodarone, 44% on digitalis and 24% on class I antiarrhythmic drugs. No significant interaction between drug effects on cycle length was observed. No significant difference in cycle length was observed between episodes with successful ATP and those with failed ATP. Comparing individual antiarrhythmic drugs in a logistic regression model, no antiarrhythmic drug conferred a significant odds ratio for ATP success or failure.
Overall 69 (39%) episodes were treated by high voltage shock, either as first line treatment or after failed ATP. All shocks were successful in terminating the ventricular tachyarrhythmia.
Inappropriate ICD shocks
Five patients (36%) suffered from inappropriate ICD shocks due to supraventricular tachycardia. A total of 31 episodes of supraventricular tachycardia were inappropriately treated by the ICD, 28 led to inappropriate shocks. The remaining 3 terminated spontaneously after one or more bursts of ventricular ATP, but before delivery of high voltage therapies. The mean ventricular cycle length was 247±29 ms, significantly shorter (P<0.001) than the cycle length of the true ventricular episodes. No significant difference was observed between systems with atrial leads and those without, neither with regard to the number of shocks for SVT per patient year nor with regard to the time to first inappropriate shock. Mean time to first inappropriate shock for SVT was 3,578 days (95% CI, 1,182–5,974 days). Patients with inappropriate therapies for supraventricular tachycardia suffered from 0.75±0.61 inappropriate shocks per year on average. Two patients suffered from inappropriate shocks due to noise caused by lead failure.
Discussion
SCD risk in congenital heart disease
Grown-ups with congenital heart disease carry an increased overall mortality and an up to 100-fold increased risk for SCD compared to the age-matched normal population (2,5). In a large population-based study of the late SCD risk after operation for several congenital defects, SCD risk varied highly among patients with different pathologies. While the SCD risk in patients with left-to-right intracardiac shunts or pulmonary stenosis was comparable to the risk of the general population, an increased incidence of SCD in patients with aortic stenosis or dTGA was observed. The incidence of SCD in this group was estimated to be approximately 5/1,000 patient-years (5). A more recent study of 936 adults with congenital heart defects reported an even higher incidence of sudden cardiac arrest (SCA) of 9.5/1,000 patient-years in dTGA and 25/1,000 patient-years in ccTGA, being the single defect with the highest risk in the study (6). As in other heart failure cohorts, ICDs proved highly effective for the prevention of arrhythmic death in the present study. However, competing risks of death in heart failure need to be considered as well, and selected patients might be candidates for mechanical support (10-12).
Appropriate ICD therapies
The largest retrospective multicenter study of ICD therapy in grown-ups with congenital heart defects to date was reported by Koyak and co-workers in 2012 (13). Of 136 patients in this study, 51% suffered from tetralogy of Fallot, 20% septal defects, and 13% (18 patients) ccTGA. Thirty-nine patients (29%) received appropriate therapies. Khairy and colleagues reported a retrospective multicenter study of 37 ICD carriers with Mustard/Senning-corrected dTGA (14). Twelve patients received appropriate therapies within a median follow-up of 3.6 years. In addition, two smaller series of patients with dTGA after atrial-switch repair and ICD were recently published. In the first series, 1 ICD discharge occurred in 12 patients with primary prevention ICD indication during a follow-up of 3.5 years (15). The other was especially remarkable for a massive burden of inappropriate shocks, reporting 34 ICD shocks in 11 patients with only one true VT/VF event (16). In the present study, ventricular tachyarrhythmias with appropriate ICD therapy occurred in 64% of patients, more than doubled compared to previous reports. This is probably due to the longer follow-up duration in our cohort. Another difference of the present study is that we included patients with dTGA as well as patient with ccTGA. The risk of SCD/SCA in ccTGA is even higher than in surgically corrected dTGA. We chose to include patients with both types of TGA in our analysis to represent the real world situation at our institution. ccTGA is rare with an incidence of approximately 1:33,000 in comparison to dTGA (1:3,500–5,000 live births), but accounts for half of the patients in our cohort (17,18).
Inappropriate therapies
Patients with TGA exhibit a high burden of supraventricular tachycardias (19). ICD carriers are therefore at risk for inappropriate shocks: In one series of dTGA patients with ICDs implanted for primary prevention, the ratio of inappropriate shocks to appropriate therapies was as high as 10:1 (16). Although the proportion of inappropriate shocks for supraventricular tachycardia was lower in our cohort, they still pose a relevant problem. Of note, fast SVTs are common and the mean cycle length for inappropriately treated SVTs was shorter than for ventricular arrhythmias. Dual chamber ICDs do not reduce the burden of inappropriate therapies, an observation which is consistent with previous reports in other populations of ICD carriers (20).
Efficacy of anti-tachycardic pacing
While ATP is highly effective in patients with ischemic or non-ischemic cardiomyopathy, the scarce data available to date suggested only marginal efficacy in patients with TGA (21-23). In the study by Koyak and co-workers, only 5 of 26 ATP attempts in patients with diverse congenital heart defects were effective (13). In contrast, ATP was highly effective in our cohort with success rates comparable to patients with ischemic or non-ischemic cardiomyopathy or other types of congenital heart disease (mainly Tetralogy of Fallot) (23,24). Mean cycle length was 277 ms in TGA patients reported by Koyak compared to 310 ms in our population. Although we observed no significant influence of the tachycardia cycle length on ATP success in our patients, shorter cycle lengths in the Koyak study could indicate a higher proportion of ventricular fibrillation or polymorphic fast VTs as opposed to monomorphic ventricular tachycardia episodes.
The observed high efficacy of ATP in these patients has profound clinical implications: First, the programming of ATP is highly recommended to avoid unnecessary shocks. Second, the choice of transvenous vs. S-ICD systems should be made with care. Depending on concomitant heart defects or prior operations such as tricuspid valve replacement, transvenous lead positioning may be complicated or impossible. In such patients the S-ICD may be preferable or may even be the only option. On the other hand, given the high efficacy of ATP, the lack of ATP capabilities is a major drawback of the S-ICD.
Study limitations
The main limitation of the present study is the sample size. This is offset by the long follow-up duration covered, resulting in the largest number of arrhythmia episodes in this patient group reported to date. The chosen design of identifying all active patients at a certain point in time, collecting retrospective data and then following them prospectively for another 2 years might be a cause of selection bias towards long-term survivors. However the current design allowed collection of a large number of ventricular tachyarrhythmias.
Conclusions
In a contemporary cohort of dTGA and ccTGA patients with ICD, we observed a high proportion of appropriate ICD therapies for ventricular tachyarrhythmias. Over a period of up to 15 years (median 5 years) almost two thirds of all patients received appropriate therapies. ATP is highly effective in this population and should therefore be programmed whenever possible. This is important when considering risks and benefits of transvenous ICDs versus S-ICDs, which might be favorable in patients with altered anatomy but lack ATP capabilities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The requirement of informed consent for observational studies was waived by the institutional committee on human research.
References
- Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 2001;37:1170-5. [Crossref] [PubMed]
- Nieminen HP, Jokinen E V, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: a population-based study. J Am Coll Cardiol 2007;50:1263-71. [Crossref] [PubMed]
- Chubb H, Rosenthal E. Implantable cardioverter-defibrillators in congenital heart disease. Herzschrittmacherther Elektrophysiol 2016;27:95-103. [Crossref] [PubMed]
- Oechslin EN, Harrison DA, Connelly MS, et al. Mode of death in adults with congenital heart disease. Am J Cardiol 2000;86:1111-6. [Crossref] [PubMed]
- Silka MJ, Hardy BG, Menashe VD, et al. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol 1998;32:245-51. [Crossref] [PubMed]
- Gallego P, Gonzalez AE, Sanchez-Recalde A, et al. Incidence and predictors of sudden cardiac arrest in adults with congenital heart defects repaired before adult life. Am J Cardiol 2012;110:109-17. [Crossref] [PubMed]
- Yousuf O, Chrispin J, Tomaselli GF, et al. Clinical management and prevention of sudden cardiac death. Circ Res 2015;116:2020-40. [Crossref] [PubMed]
- Coskun KO, Popov AF, Schmitto JD, et al. Feasibility of implantable cardioverter defibrillator treatment in five patients with familial Friedreich’s ataxia--a case series. Artif Organs 2010;34:1061-5. [Crossref] [PubMed]
- Gold MR, Theuns DA, Knight BP, et al. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol 2012;23:359-66. [Crossref] [PubMed]
- Lund LH, Trochu JN, Meyns B, et al. Screening for heart transplantation and left ventricular assist system: results from the ScrEEning for advanced Heart Failure treatment (SEE-HF) study. Eur J Heart Fail 2018;20:152-60. [Crossref] [PubMed]
- Uribarri A, Rojas SV, Avsar M, et al. First series of mechanical circulatory support in non-compaction cardiomyopathy: Is LVAD implantation a safe alternative? Int J Cardiol 2015;197:128-32. [Crossref] [PubMed]
- Duncker D, König T, Müller-Leisse J, et al. Electric smog: telemetry interference between ICD and LVAD. Herzschrittmacherther Elektrophysiol 2017;28:257-9. [Crossref] [PubMed]
- Koyak Z, de Groot JR, Van Gelder IC, et al. Implantable cardioverter defibrillator therapy in adults with congenital heart disease: who is at risk of shocks? Circ Arrhythm Electrophysiol 2012;5:101-10. [Crossref] [PubMed]
- Khairy P, Harris L, Landzberg MJ, et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol 2008;1:250-7. [Crossref] [PubMed]
- Backhoff D, Müller M, Ruschewski W, et al. ICD therapy for primary prevention of sudden cardiac death after Mustard repair for d-transposition of the great arteries. Clin Res Cardiol 2014;103:894-901. [Crossref] [PubMed]
- Buber J, Ackley TJ, Daniels CJ, et al. Outcomes following the implantation of cardioverter-defibrillator for primary prevention in transposition of the great arteries after intra-atrial baffle repair: a single-centre experience. Europace 2016;18:1016-22. [Crossref] [PubMed]
- Wallis GA, Debich-Spicer D, Anderson RH. Congenitally corrected transposition. Orphanet J Rare Dis 2011;6:22. [Crossref] [PubMed]
- Martins P, Castela E. Transposition of the great arteries. Orphanet J Rare Dis 2008;3:27. [Crossref] [PubMed]
- Warnes CA. Transposition of the great arteries. Circulation 2006;114:2699-709. [Crossref] [PubMed]
- Lawrence D, von Bergen N, Law IH, et al. Inappropriate ICD discharges in single-chamber versus dual-chamber devices in the pediatric and young adult population. J Cardiovasc Electrophysiol 2009;20:287-90. [Crossref] [PubMed]
- Duncker D, Veltmann C.. Optimizing Antitachycardia Pacing: Back to the Roots. Circ Arrhythm Electrophysiol 2017;10:e005696. [Crossref] [PubMed]
- Saeed M, Neason CG, Razavi M, et al. Programming antitachycardia pacing for primary prevention in patients with implantable cardioverter defibrillators: results from the PROVE trial. J Cardiovasc Electrophysiol 2010;21:1349-54. [Crossref] [PubMed]
- Santini M, Lunati M, Defaye P, et al. Prospective multicenter randomized trial of fast ventricular tachycardia termination by prolonged versus conventional anti-tachyarrhythmia burst pacing in implantable cardioverter-defibrillator patients-Atp DeliVery for pAiNless ICD thErapy (ADVANCE-D) Tr. J Interv Card Electrophysiol 2010;27:127-35. [Crossref] [PubMed]
- Kalra Y, Radbill AE, Johns JA, et al. Antitachycardia pacing reduces appropriate and inappropriate shocks in children and congenital heart disease patients. Heart Rhythm 2012;9:1829-34. [Crossref] [PubMed]


