Biomaterials and heart recovery: cardiac repair, regeneration and healing in the MCS era: a state of the “heart”
Introduction
Cardiovascular diseases (CVD) represent, nowadays, a relevant cause of mortality and morbidity all over the word. Approximately 17.8 million people died from CVD in 2015 and this number is estimated to reach to 22.2 million by 2030 (1).
The acronym CVD defines several disorders affecting the cardiovascular system comprising heart failure (HF) and heart valve disease (HVD).
In this context, regenerative medicine and tissue engineering, holding the potential to overcome a wide range of limitations, can be both considered innovative and valid alternatives to standard therapeutic approaches.
Commonly, HF is caused by repeated coronary artery occlusion [ischemic heart disease (IHD)] progressively reducing the amount of functional myocardium. With the increasing survival to the first event due to the availability of pharmacological and interventional reperfusion the incidence of IHD has become the most common cause of HF and indeed one of the most common cause of death. However, due to poor late or absent post-ischemic reperfusion of the infarcted myocardial areas, a significant number of cardiomyocytes undergoes necrosis and is replaced by fibrous scar tissue. This complex replacing process rapidly leads to several changes in the native structure of the cardiac extracellular-matrix.
The relative unpredictability of acute cardiovascular events, the uncertain reduced 5-year mortality rate even shifting from coronary artery bypass graft (CABG) to promptly available early percutaneous coronary intervention (PCI) reperfusion (2,3) and the absence of a durable post-ischemic treatment, has endorsed IHD to one of the most critical clinical hurdle of the previous century and postinfartual HF as the real challenge for the ongoing century. The long-term survival of patients with HF is compromised by a series of complications as the massive proliferation of non-contractile fibrotic tissue in the infarcted areas. Additionally, after acute ischemic episodes, patients endure life-changing treatments ranging from daily medications to surgical interventions, e.g., pacemakers, stents, angioplasty or heart transplants (4).
To improve quality of life and life expectancy in patients with CVD preclinical research is leading us to new therapeutic approaches. Hopefully, over the next years, much effort will be aimed to promote the formation of functional contractile tissue in the scar areas of ischemic heart.
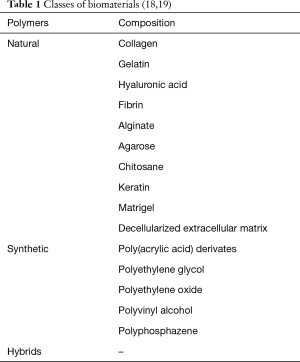
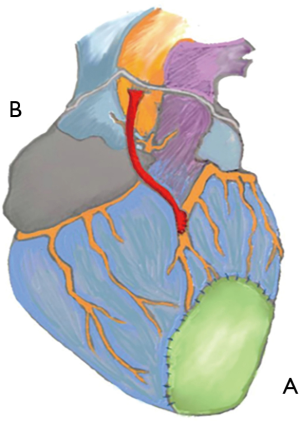
Since the beginning the central focus of regenerative medicine has been using stem cells (i.e., embryonic stem cells (ESCs), adipose tissue- or bone marrow- or skeletal muscle-derived mesenchymal stem cells, endothelial progenitor cells derived from peripheral blood and cardiac progenitors) to restore organ function; two possible approaches have been explored: the surgical approach and the intracoronary approach (Figure 1). Cell therapies delivering single-cell suspensions of autologous skeletal myoblasts, bone marrow cells, or sorted subpopulations into the myocardium or coronary arteries after myocardial infarction (MI) have proven safe but may lack efficacy (5). This may be due to the limited ability of these cells to survive and convert into fully mature contractile cardiomyocytes (6).
Surprising results have been reached stimulating the endogenous cell populations resident in the infarcted myocardium.
Actually, it is possible to induce the cardiac extracellular-matrix remodelling and substitution using hybrid hydrogel injections or synthetic or biologic cardiac patches.
Furthermore, there are accurate evidences on the possible use of tissue engineered vascular grafts (TEVGs) and bioresorbable stents in patients undergoing CABG or coronary angioplasty, respectively.
Therefore, we promote switching our attention on the contribution given to Regenerative Medicine by Tissue Engineering, improving the sapient use of scaffolds and biomaterials, stimulating the autologous potential still present in the damaged tissue. We do not exclude the possibility to implant cell seeded devices (i.e., TEVGs), if necessary.
We will describe the most relevant achievements in CVD therapies obtained by influencing tissue biology and mechanics using biomaterials.
New options to avoid ischemic cardiomyopathy
During a heart attack, cardiomyocytes undergo necrosis and myocardium is replaced by scar tissue over the course of several weeks. Myocardial wound healing cascade following infarction is a dynamic and complex process wherein a variety of inflammatory cells (neutrophils, macrophages and lymphocytes) and activate matrix metalloproteinases (MMPs) are involved.
Inflammatory cells upregulate the release of a myriad of signaling cytokines, growth factors, and hormones including transforming growth factor β (TGF-β), interleukins 1, 2, 6, and 10, tumour necrosis factor α, interferon γ, chemokines of the CC and CXC families, angiotensin II, norepinephrine, endothelin, natriuretic peptides, and platelet-derived growth factors.
MMPs degrade cell and matrix material aiding phagocytic macrophages in the resorption of necrotic tissue. Rapidly MMPs disrupt the collagen fibers and struts that supported cardiomyocyte structure in the once-healthy myocardium (7).
It has been estimated a loss of 50% of ECM collagen within the first 3 h post-ischemia (8).
While new collagen accumulation is not apparent for several days after the initial injury, as necrotic myocytes are resorbed, a provisional granulation tissue consisting of fibrin, fibronectin, laminin, glycosaminoglycans (GAGs), and other matrix is laid in their place within 24 hours after infarction until the fibrotic and remodelling phases when fibrillar pro-collagen type I and III ramps up such as the quantity of ECM molecules cross-links. Damaged myocardium stops contracting and begins stretching and recoiling along a curve that reflects passive properties. Fibrillar collagen, cross-linking and ECM changes are the determinants of those passive properties. Both collagen content and collagen fibers orientation are critical determinants of the mechanical properties of healing infarct scars.
At the end of this process the ischemic scar tissue lost a great quantity of collagen fibers, has an increased number of cross-links, lost its elasticity, become mechanically weakest and may undergo left ventricle (LV) dilatation or cardiac rupture.
The size, location, composition, structure and mechanical properties of the healing scar are all critical determinants of the fate of patients who survive the initial infarction. Scar structure and remodelling process have been recognized determining factors influencing pump function (7).
The remodelling of the LV is a compensatory response that can be initiated following MI, where wall thinning, chamber dilation and shape modification occur to maintain cardiac output by increasing the stroke volume (8).
It has proven remarkably difficult to design therapies that improve heart function or limit remodelling by modifying scar structure (7).
Approaches developed to mitigate LV remodelling include drug treatments [e.g., beta blockers + acetylcholinesterase inhibitors (AchEI)] (9).
Angiotensin converting enzyme inhibitors (ACEIs) and β-blockers are the primary approach in patients at high risk for HF with or without structural heart disease and have been shown to reduce mortality and morbidity in patients with HF (10).
This pharmacological approach has met with success in several patient populations but progression towards end-stage HF still occurs.
The β-blockers were reported to increase collagen content ~60% in 12-week-old rat infarcts (11) but conversely the ACEI resulted in a significant decrease (~45%) of collagen content at 28 days post-MI in a rat model (12).
Even different surgical procedures [e.g., Dor (13), Batista (14)], and epicardial restraint devices (e.g., Acorn (15), Paracor (16)] were not able to avoid the long-term progression of the disease.
Recently, ventricular assist devices (VADs) have showed the capability to promote end-stage failing hearts to recover and reverse remodeling with functional improvement, allowing VAD removal followed by years of freedom from HF recurrence. At cellular and subcellular level myocardial recovery has been observed after VAD implantation, while translation of these changes into functional recovery at organ level was observed less frequently, and stable cardiac improvement with long-term HF-free outcome after VAD removal has rarely occurred. However, in the acute phase LV assist device (LVAD) support can reverse HF. The possibility of cardiac recovery during long-term mechanical circulatory support (MCS) rises to three notable challenges: (I) search for additional therapies providing the basis for future use of VADs joined to regenerative strategies to facilitate patient recovery and to increase the number of weaning candidates; (II) assessment of recovery after VAD implantation and decision making on VAD explantation; and (III) preimplantation prediction of possible cardiac recovery during MCS (17).
As interventions to abrogate ischemic cardiomyopathy, the concept of injecting Hydrogels in the scar tissue or of applying a temporary, local patch to the surface of the recently infarcted ventricle have been explored.
Hydrogel injection are, nowadays, clinically tested in patients within a week from primary PCI or complete revascularization and in patients with advanced chronic HF, while Cardiac patches are still under preclinical testing.
The use of injectable hydrogels post-MI
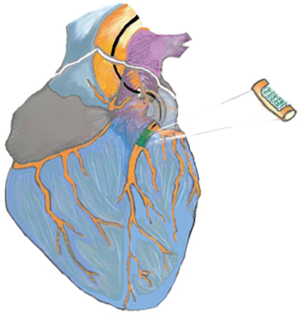
Hydrogels injection for cardiac tissue repair is a promising therapeutic approach in MI treatment (Figure 2). Hydrogels have been effective in MI, limiting extension and expansion of ischemic injury, resulting in improved survival rates.
Numerous hydrogels have been synthesized for tissue engineering but only a few of them have been successfully tested for cardiac applications. However, the most relevant goal of new hydrogel materials is the possibility to inject them via transcatheter procedure, even if the range of materials available for percutaneous approach is still limited.
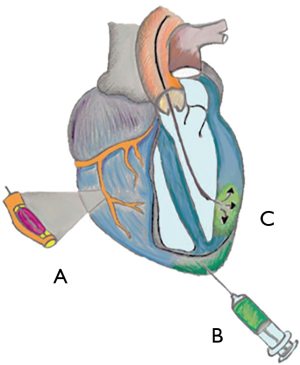
These injectable hydrogels are surprisingly useful as cells or drugs delivery vehicles and can be made of a great variety of biomaterials (Table 1) (18,19).
The optimal biomaterial should be easy to manufacture, inexpensive to produce, biocompatible, biodegradable, non-toxic, minimally immunogenic, and should possess mechanical properties similar to the target tissue (20). Another critical property is low viscosity to permit the material to be injected, contrasting with its efficacy. In situ rheology of the injected material should exhibit viscoelastic properties to prevent washout from the target zone.
Several works have shown that these materials can provide maintenance or improvement in functional parameters in small animals and large-mammalian models either alone, or with cells or growth factors.
Acellular hydrogels without any therapeutic biomolecules were found to thicken the myocardial wall, thereby reducing abnormal stresses when injected after a MI (21,22). It has been reported that injectable hydrogels can be used as carriers for various biomolecules (23) and cells (22,24,25), enhancing cardiac function, reducing infarct size, and inducing neovascularization (Table 2).
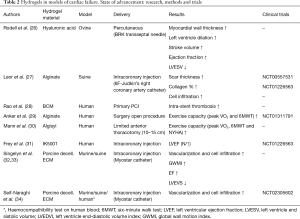
Full table
Rodell et al. (26) developed in a preclinical ovine model, a hyaluronic acid (HA) hydrogel a stiff-dual cross-linking (DC) hydrogel suitable to be delivered via percutaneous intramyocardial injection. The primary constituents (i.e., HA and DC) are generally recognized as safe by the FDA and are industrially well represented in the pharmaceutical and medical device industries (35,36). The defined material formulations, constitute a medical device that holds potential to rapidly progress toward clinical use. They are both shear-thinning and self-healing. Their injection showed greater reduction in myofiber stress, relative to MI controls with ejection fraction (EF) modeling, driven by the preservation of LV shape by maintaining the LV wall thickness. DC hydrogel injection treatment resulted in significant reduction in LV end-systolic volume (LVESV), demonstrated a consistent improvement in EF and in stroke volume, indicating preservation of ventricular geometry and function (26).
Alginate hydrogels act as a permanent prosthetic scaffold that aim to reduce wall stress, and prevent further LV enlargement based on Laplace law and has been investigated in several clinical trials.
Leor et al. demonstrated success with intracoronary infusion of alginate in pre-clinical work in swine (27) now valuated in a phase I/II study in acute MI patients (NCT00557531), and an ongoing phase II clinical trial (NCT01226563).
In a prospective comparison of alginate-hydrogel with standard medical therapy (AUGMENT-HF), Anker et al. (29) determined the impact on functional capacity and clinical outcomes of the Alginate-hydrogel (Algisyl) in patients with advanced HF (NCT01311791). This international, multi-centre, prospective, randomized, controlled trial evaluated the benefits and the safety of a novel therapeutic approach based on alginate-hydrogel in patients with advanced chronic HF. Alginate-hydrogel in addition to standard medical therapy for patients with advanced chronic HF was more effective than standard medical therapy alone for improving exercise capacity and symptoms.
Alginate-hydrogel device is implanted with relative ease in the context of a limited surgical procedure under general anaesthesia. The primary end-point of the trial was the peak VO2 values, had a 1.24 mL/kg/min change in peak VO2 over 6 months, representing a 10.2% improvement over baseline in this selected population. The 6MWT distance in the alginate-hydrogel group at baseline was below the 300 m and improved >100 m, at the 3- and 6-month follow-up visits. The NYHA functional class at 6 months was improved (84% NYHA class I or II vs. 26% in the Control group). The results of the AUGMENT-HF trial provide proof of concept for this new treatment approach even though require validation and extension in larger patient cohorts to be followed for longer periods of times with a more insightful endpoint (29).
Mann et al. demonstrated that Algisyl administration by limited anterior thoracotomy could provide sustained 1-year benefits in exercise capacity, symptoms, and clinical status for patients with advanced HF reducing cardiac mortality and re-hospitalizations.
A merely functional evaluation of clinical features (peak VO2, 6MWT and NYHA class) seems however inadequate to evaluate the effects of the injection due to the large number of determinants affecting functional capacity of patients suffering HF (30).
Frey et al. tested for the first time in a clinical trial (NCT01226563) the feasibility of intracoronary delivery of an innovative bioabsorbable alginate hydrogel (IK-5001) to prevent or reverse adverse LV remodeling and dysfunction in patients after ST-segment elevation MI (STEMI). IK-5001 has been administrated through the infarct-related coronary artery within 7 days after MI. During a 6-month follow-up they confirmed by clinical assessments, echocardiographic studies, 12-lead electrocardiograms, 24-hour Holter monitoring, blood tests and completion of Minnesota Living with Heart Failure, favourable tolerability od the procedure without adverse events related to the injection and a preservation of left ventricular indices and LV EF (31).
Even Rao et al. tested the IK-5001 in an international, randomized, double-blind, controlled trial, which included 303 subjects with large areas of infarction despite successful primary PCI of STEMI. IK-5001 was intracoronary injected into the infarct-related artery 2 to 5 days after primary PCI. The primary outcome was change in LV end-diastolic volume index (LVEDVI) at 6 months, that appears a valuable end-point to ascertain the effectivity of injection in terms of rebuilding tissue ultrastructure.
Furthermore, intracoronary deployment of BCM 2 to 5 days after successful reperfusion in subjects with large MI did not reduce adverse LV remodeling or cardiac clinical events at 6 months. The disclosed benefit in change in 6MWT from baseline to 6 months underline functional evaluation multifactoriality. Regarding individual safety endpoints, there were numerically lower rates of death and MI at 6 months with BCM but there were numerically higher rates of stent thrombosis, target-vessel revascularization, and significant arrhythmia requiring therapy with BCM (28).
So that, even if the alginate hydrogel itself is considered as an ECM biomaterial and, ECM-based materials have emerged as a promising class of naturally derived biomaterial scaffolds, given the tissue a specific ECM could be a solution to the uncertain long-term advantages of the injective therapy. It follows that the most appropriate scaffold to replace the damaged ECM after a MI would be derived from myocardial tissue.
It is interesting that Singelyn et al. developed an injectable ECM hydrogel derived from decellularized porcine myocardial tissue (32), which self-assembles into a porous and fibrous scaffold upon injection into tissue, and recently demonstrated that injecting this ECM hydrogel post-MI in a rodent model mitigated LV remodeling and preserved EF (33). Do to the shortage of human tissue, considering that it has greater variability and the limited collection of healthy human hearts, it was used porcine-derived matrix the most viable source as a clinical product and already approved by the FDA (37).
However, to translate this material to the clinic, it was necessary to address the biocompatibility and safety of this ECM-derived material and to investigate efficacy in a clinically relevant large-animal model. In a recent study, Seif-Naraghi et al. used VentriGel, an injectable ECM hydrogel derived from decellularized porcine myocardial tissue to reverse the negative remodelling process in infarcted myocardial tissue in pigs (34).
Upon injecting, this hydrogel self-assembles into a porous scaffold and allows cell infiltration. Histological characterization showed that the ECM hydrogel developed a distinct layer of endocardium, a cardiomyocyte population was identified in the infarcts of matrix-injected pigs, the hydrogel reduced myocardial fibrosis and could induce foci of neovascularization. Furthermore, myocardial matrix does not adversely affect peripheral tissues, cardiac rhythm or blood chemistry in pigs, it does not cause embolization or ischemia; it is biocompatible, biodegradable and human blood tests show hemocompatibility.
The myocardial matrix has been the second biomaterial to be delivered percutaneously in a large MI model and the first via a transendocardial approach. Transendocardial surgical delivery is considered to be the preferred method of catheter delivery (38). It allows direct intramyocardial delivery increasing biomaterial retention, not requiring access to the coronary vessels and reducing the risk of embolization. Additionally, the coronary access required for intracoronary delivery is often compromised in the target patient population.
According to the potential for ventricular rupture, due the unstable ventricular wall caused by MMP upregulation in the first week post-MI, early injection procedure is generally considered clinically risky. Moreover, injection immediately post-MI would cause premature degradation of ECM-derived scaffold as the native ECM. So that Seif-Naraghi et al. performed the injection within 2 weeks from the MI.
In the matrix-injected animals the decreased LVESV, the rise of the EF and the improved global wall motion index (GWMI), which is a measure of regional function, a significant predictor of cardiovascular death after acute MI and an independent prognostic value when added to a model that includes EF and other baseline clinical values, demonstrated a positive change in myocardial function (34). There were found improvements in global and regional cardiac function and LV volumes, whereas alginate, the only other material to be delivered percutaneously in a large MI animal model, significantly changed LV geometry, but not fractional shortening (27). It has to be considered that actually the only hydrogel alone, which seems to be able to modulate the EF and functional shortening, was HA (26).
Finally, Seif-Naraghi et al. in their preclinical study (34) provided support for the advancement of this technology from preclinical studies to a first-in-human study. The results of the clinical trial on VentriGel will be available since 2018 (NCT02305602).
The use of cardiac patches post-MI
Contrasting with the research advancement on hydrogels clinical employment there are no clinical trials on the possible use of cardiac patches (Figure 3A) (Table 3), even though the biodegradable cardiac patch developed by D’Amore et al. still in preclinical study, seems capable to give appropriate mechanical support to the fibrous scar and to influence the remodeling pathway providing cellular or biomolecule delivery.
Full table
It is a bi-layered scaffold made by biodegradable [poly(ester carbonate urethane)urea (PECUU)] with isotropic mechanics, incorporating an epicardial-facing cardiac ECM enriched layer. In D’Amore et al. murine model, the sterile non-immunogenic, isotropic and bio-hybrid patch, produced using a modified two stream electrospinning process (39), was easily affixed to the epicardial surface of infarcted LV. Epicardial surface was lightly scarped (removing both fibrotic tissue and remnant epicardium to a level <100 µm) at the patch implant site. Next, the patch was affixed with the ECM side directly facing the epicardial region on the anterior infarcted myocardium using 7-0 polypropylene with over and over peripheral continuous sutures. The ECM patch treatment increased LV anterior wall thickness to ~2,300 µm. This result represents an incremental improvement in outcomes when compared with previous reports on rat chronic MI models.
This patch induces a significant increase the ventricular wall stiffness under biaxial loading and mitigates wall thinning preventing chamber dilation and reducing wall stress.
For instance, Hashizume et al. (40) adopted elastomeric polyurethane patches fabricated by salt leaching without ECM and reported a LV wall thickness of ~1,300 µm, Lin et al. (41) utilized electrospun synthetic bioresorbable cell-seeded engineered construct and achieved a LV wall thickness on the patch treated group of ~1,000 µm.
So that compared to no ECM-patches, even if cell-seeded, there is an improved thickening of the LV anterior wall with patches containing the ECM rich layer. This result may reach benefit from both the mechanical support provided by the polymer rich layer and the endogenous cellular infiltration putatively stimulated by the ECM gel presence.
The presence of the ECM was associated with endogenous tissue growth within the patch and marked improvement in host cell recruitment, promoted a positive effect in terms of inflammatory cells regulations, inducing an M2 versus M1 macrophage phenotype, and stimulated remarkable angiogenesis with the formation of new capillary well perfused networks inside and underneath the patch treated regions.
The benefit of designing a cardiac patch that combines ventricle mechanical support, through a biodegradable, fibrillar elastomeric component, and biological interactions by the incorporation of ECM-based hydrogel components, is demonstrated.
The hybrid patch can be considered as a valid compromise between the decellularized cardiac ECM alone difficult to suture and apply on the damaged tissue and not able to offer the best mechanical support, and the non-ECM containing patches that cannot produce significant effects on echocardiographic function, cell bioactivity and wall thinning or scar formation, even though the whole organ pressure-volume characterization, as well as the LV wall biaxial mechanics demonstrated a supportive stiffening effect.
According to the encouraging results obtained, these preclinical studies, even if the rat model is ideally suited to investigating manipulations in patch design, should be extended to a large animal model producing patches similar in size to what might be clinically applied; furthermore, detailed studies on the mechanism by which the tissue functional restoring is achieved, are required.
Shadrin et al. developed the first method to generate a large (4 cm × 4 cm) and functional Cardiopatch that meets the “safety and efficacy” requirements for human cardiac repair. This patch supports fast action potential conduction to reduce risk of arrhythmias, produces strong contractile forces to aid in mechanical pumping of native heart, is sufficiently large to cover entire infarcted area, and undergoes vascularization to promote long-term survival; it has been obtained in 5 weeks combining a specific hydrogel-molding with a dynamic human embryonic and induced pluripotent stem cells (hPSC/iPSCs) culture to develop a versatile in vitro platform for rapid maturation of 3D engineered Cardiopatch without need for exogenous stimulation. Compared to PCI cell/hydrogel injection strategies currently tested in clinics, surgical implantation of engineered functional cardiac tissues could offer greatly improved survival, retention, and paracrine action of implanted cells at the infarct site, adding structural support, and antiarrhythmic effects from full-length coverage of the scar with electrically conducting tissue (42).
Menasché et al. demonstrated improved cardiac function in a 68-year-old patient with advanced HF following epicardial implantation of a 20 cm2 fibrin-based tissue patch containing hESC, providing a foundation for future use of hPSC-based strategies in human heart repair (43).
All these patches still require thoracotomy for epicardial placement, we may overcome this limit considering cardiac patches as local restrain devices in patients with chronic HF, so that the clinical testing of such devices can be performed on patients at the end-stage pathology where mechanical unloading (e.g., LVADs) is required, or patients undergoing invasive coronary artery by-pass grafting.
New options in cardiac revascularization
In the US alone, approximately 400,000 CABG are performed annually. During the past decade, there has been nearly a 30% decline in CABG procedures, despite aging population and growing evidence to support surgery effectiveness and safety (44). This decline has been accompanied by an increase in PCI revascularization. Over 1,000,000 cardiac catheterizations are performed annually in US (45). Hence, significant improvements in the technology of coronary stents and vascular grafts is necessary to meet the clinical demand.
Vascular engineered grafts
Synthetic grafts (Figure 3B) demonstrate good patency and long-term outcomes for management of the aorta and other large arteries (>5 mm diameter), but their use for bypass of small diameter vessels has been associated with less than satisfying results due to the high frequency of thrombosis, stenosis, occlusion and infection (46).
Autologous tissue, such as internal thoracic artery or saphenous vein, is the first choice for small diameter arterial grafts. However, prior surgeries and patient’s medical conditions may limit the availability of autologous vessels, and their harvest can be associated with significant morbidity (47). Even with patient’s own grafts CABG, long-term outcomes are negatively impacted by high rates of thrombosis and intimal hyperplasia. Therefore, there remains a substantial unfulfilled need for readily available small-calibre vascular grafts with good long-term patency (48).
Logistically, ideal TEVGs have to maintain balance between degradation and neo-tissue formation, they should be biomimetic, biocompatible, no toxic or excessively immunogenic, should resist to long-term complications, such as infection, intimal hyperplasia, stenosis, calcification, and aneurysmal dilatation, should be resilient, must minimize production time, and in the case of “off-the-shelf” unseeded TEVGs should allow storability (49).
Various strategies for delivering or embedding pharmacological agents within the graft have been investigated to enhance their biocompatibility and functional properties, adding the drug and/or cells directly into the biomaterial used to produce vessels or grafts
Scaffold for TEVGs capable of neo-tissue formation can be produced, by electrospinning techniques (50,51) employing biodegradable polymers offering different chemical and mechanical proprieties (i.e., molecular weight, surface-area-to-volume ratio, crystallinity, tissue hydrophily, specific degradation time, retention strength and, shear force and tension resistance), obtaining self-assembly scaffolds or hydrogels, that enhance biocompatibility and cellular recruitment, or a combination of degradable synthetic and natural polymers producing hybrid scaffolds (Table 4).

Full table
The presence of native proteins promotes a more native-like immediate microenvironment and allows cellular signaling. TEVGs attempt to recapitulate the functions of each component in the vessel by promoting cellular infiltration and proliferation to facilitate the synthesis of these proteins (58,59) (Table 5).

Full table
There have been different cell types, usually autologous, used in cell seeding (Table 6) (70) endothelial progenitor cells (EPCs) from human peripheral blood (61,62).

Full table
Mesenchymal stem cells (MSCs) are isolatable from peripheral blood (66), bone marrow by aspiration (62,63), skeletal muscle by muscle biopsy (64) and adipose tissue by liposuction (65). MSCs could be harvested from a patient, expanded in vitro, and used for a personalized TEVG (65).
Krawiec et al. developed a culture-free method of differentiating MSCs obtained from liposuction into smooth muscle cells (SMCs) that were used in TEVGs (71).
ESCs have the potential to differentiate into any adult cell type. For this proliferative capability and versatility the result appealing, but it is hard to induce commitment during their differentiation process and, in addition to ethical considerations, they are potentially tumorigenic. In Sundaram et al. mature graft, ESCs differentiated into osteoblasts and chondrocytes expressing bone and cartilage markers (67).
iPSCs (68) from skin or blood should be an alternative to ESCs. Hibino et al. demonstrated that mouse iPSCs could be seeded, with good endothelialization, on PLGA and poly-ε-caprolactone-co-L-lactide (PCLA) scaffolds in a mouse inferior vena cava model (72). Gui et al. and Sundaram et al. used human iPSCs in an aortic interposition model in nude mice with good outcomes such as no ruptures, stenosis, or teratomas at 2 weeks (73,74). However, the number of seeded differentiated iPSCs decreased over time (42% at 1 week, 10% at 4 weeks and 10 weeks) (72).
Davoudi et al. defined essential, in vascular TE, efficient endothelialization of graft surfaces through cells adhesion, spreading and proliferation. Synthetic polymers do not provide optimum interaction with cells to promote endothelialization. They propose an electrospun nanofibrous scaffolds of polyurethane (PU) modified with a layer of polydiacetylene (PDA) consequently, coated with heparin and vascular endothelial growth factor (VEGF). These modifications not only improved the mechanical and surface properties of the scaffolds, but also enhanced human umbilical vein endothelial cells (HUVECs) adhesion, spreading and proliferation on the scaffolds. The functional scaffolds demonstrated appropriate blood compatibility and is to be considered as potential improvement for small caliber vascular grafts and cardiovascular tissue engineering products (69).
Until today the use of autologous cells, even if easily collected, requires longest wait times, up to months (75), and hasn’t reached the efficacy required by surgical timing. The major impediment to the clinical implementation of TEVGs is the waiting time for graft creation. Acellular grafts, such as the Humacyte human acellular vessel (HAV) (76) are also helpful in decreasing waiting times.
Dahl et al. cultured human/dog MSCs on a PGA scaffold that was subsequently decellularized, and tested them in models of coronary bypass and AV grafts which demonstrated good functional outcomes and showed progress towards “off-the-shelf” (75-77). However, in low pressure systems, Hibino et al. demonstrate a technique for harvesting bone marrow, isolating cells, seeding them, and implanting them on the same day (78).
Even if it is necessary to improve knowledge on biomaterials and cell seeding on TEVGs, these devices show great promise in all fields of vascular surgery (Table 7), especially in paediatric cardiac surgery, where patients have to undergo repeated surgeries as their circulation systems outgrow implanted conventional grafts of fixed diameters (81), there are several reports of clinical trials for TEVGs. Shin'oka et al. and Hibino et al. reported a clinical trial of PGA/PCL or PLA grafts used for extracardiac cavopulmonary shunts in children. At a mean follow-up interval of 5 years, graft stenosis was the primary mode of graft failure even if they had no evidence of aneurysm, rupture or calcification (79,80). Bockeria et al. also performed a clinical trial of vascular grafts {PCL with 2-ureido-4[1H]-pyrimidinone (UPy) motif} as extracardiac cavopulmonary conduits in 5 paediatric patients undergoing Fontan surgery. At 1 year, there were no device related adverse events and the implanted grafts demonstrated stable conduit diameters, lengths, wall thicknesses and blood flow patterns (81).
Full table
Lawson et al. performed two phase II studies of their decellularized PGA scaffold TEVG (seeded with MSCs from donors) as upper limb arteriovenous grafts in 60 renal patients requiring haemodialysis and demonstrated safety (1 infection in 82 patient-years of follow up) and efficacy (patency) (83).
McAllister et al. have also conducted a multi-center cohort study of the effectiveness of hemodialysis access for renal patients using TEVGs, and found that their primary patency rate approaches established quality objectives for arteriovenous (AV) fistulas (82).
Lawson et al. are assessing safety and efficacy of a novel, tissue-engineered vascular prosthesis, the human acellular vascular graft (HAVG). It is intended as an alternative to synthetic materials and to autologous grafts in the creation of vascular access for dialysis (NCT01840956) (86). Now it is even compared with polytetrafluoroethylene (ePTFE) grafts used for hemodialysis access (NCT02644941) (84). Another autologous-biological tissue engineered blood vessel (TEBV, Lifeline™) used as an arteriovenous fistula for dialysis access was tested by de la Fuente et al. (NCT00850252) but no results are available (87).
More recently, the same group followed up with a study using TEVGs built from allogeneic fibroblasts implanted as brachial-axillary AV shunts for three patients requiring haemodialysis access (88).
Various biodegradable scaffolds are undergoing clinical evaluation for the treatment of coronary artery disease (89).
Morris et al. are evaluating the 6 mm Gore-PROPATEN® graft (heparin-coated ePTFE vascular graft, thin-walled removable-ring stretch) coated with an autologous coating of adipose-derived stromal cells (ASC) (NCT01305863). A Peripheral Vascular Graft (PVG) Kit consisting in a vascular conduit, a proprietary enzymatic solution and a disposable fluidics system, all to be used in conjunction with a cell isolation system (CIS), is used to prepare a stem cell-based biological coating in about an hour; it is an automated tissue processing instrument which isolates ASCs from a lipoaspirate specimen and permit to seed them onto the internal lumen of the vascular graft before it is implanted into the patient. Effectively, it can be used to address the pressing medical need for small-diameter vascular grafts with improved long-term patency rates comparable to the autologous saphenous vein (85).
Bioresorbable scaffolds (BRS) in routine PCI
BRS (Figure 4) have the potential to overcome the last remaining issues in the treatment of coronary artery lesions, which are still present with metallic stents. By providing only temporary vessel wall support, the technology could allow restoration of vessel wall physiology, vasomotion and vessel geometry after degradation of the device. One of the major potential benefits is the further reduction of late and very late scaffold thrombosis juxtaposed to second- and third-generation drug-eluting stents (DES), because in theory, resorption leaves no nidus for late scaffold thrombosis. However, more clinical data are needed to establish whether these potential benefits will lead to a patient benefit and more investigation is necessary whether the degradation products itself may cause an inflammatory response itself a cause intraluminal thrombosis, even after complete degradation of the device. Current scaffolds still have limitations, most importantly all BRS have significant thicker struts (150–200 mm) compared with newer generation metallic DES (~80 mm). This is accompanied with a more difficult delivery profile and a potential risk of increased restenosis with overlapping scaffolds that could also be more prone to delayed endothelialization (90). The increased strut thickness also raised concerns about the use of this technology in coronary artery bifurcation lesions requiring a two-stent technology and the risk of side-branch occlusions (91). Moreover, strut disruption after overexpansion of polymeric scaffolds could lead to complications such as thrombosis. A potential point of concern is that these devices could have a higher thrombogenicity, increasing the risk of acute or subacute scaffold thrombosis, on the short-term, given the strut thickness and slightly delayed coverage compared to new-generation DES (92).
Neointimal proliferation and strut coverage could be altered by changes in endothelial shear stress after implantation of BRS with the relative thick struts (93,94).
At present, the safety and efficacy of the ABSORB everolimus eluting bioresorbable vascular scaffold (BVS) has been evaluated extensively. It is, nowadays, the most evaluated BRS and is increasingly used in clinical practice.
However, most clinical data have been collected in relatively simple coronary artery lesions. Furthermore, several trials of the ABSORB BVS are ongoing. The data confirm that in more complex lesions and patients, the scaffold shows a good efficacy, with a low number of target vessel or target lesion revascularization at mid-term follow-up. However, some concerns are being raised about the safety of this device due to initial experience reports of acute and subacute scaffold thrombosis (95,96).
In the preliminary report of the AIDA trial, by Wykrzykowska et al. involving patients, there was no significant difference in the rate of target-vessel failure between patients, who undergone PCI and received a BRS, and the patients who received a XIENCE metallic stent but the BRS was associated with a higher incidence of device thrombosis than the metallic stent through 2 years of follow-up (NCT01858077) (97).
The DESolve BRS (Elixer Medical Corporation, Sunnyvale, CA, USA) is the second BRS that achieved the CE mark in May 2013, is commercially available since January 2014, and is designed to resorb in about 1 year. This stent is now on comparison with the ABSORBE in the BIFSORB study (NCT02973529) (98).
The REVA scaffolds family was first made up of a tyrosine-derived polycarbonate polymer with a unique ‘slide and lock’ design, which acts like a ratchet after balloon inflation, is radiopaque and is coated with the antiproliferative drug sirolimus to overcome the shortcomings of the first-generation scaffold (RESTORE-II study) (99).
The XINSORB BRS is a balloon-expandable BRS system composed of PLLA as its backbone and the antiproliferative drug sirolimus eluted from the matrix. Its evaluation is expected in an upcoming first-in-man trial.
Magnesium-based BRS are manufactured by Biotronik. These devices have the same mechanical force as no-resorbable metallic stents and are resorbed within 1 year (100-102).
To date (Table 8), the true clinical value of bioresorbable technology and whether it can replace metallic DES in all patient subsets is part of an ongoing debate (Figure 4).

Full table
Therefore, there is an urgent need for randomized controlled data comparing the safety and efficacy between BRS and second-generation DES.
Conclusions
HF due to any etiology is becoming a pandemic disease in the developed world. Many drugs, interventional and surgical approaches are capable to keep the patients alive until the end-stage phase. Recently, MCS has been used to revert HF. It can be used as bridge to recovery that can stimulate heart healing and reverse myocardial remodeling (103).
In the upcoming future, a large number of biomaterials for hydrogels, patches, TEVGs and BRS are going to be available in the clinical arena. Many of these have been successfully implanted in preclinical studies and several trials have been registered to investigate the effectiveness of such regenerative approaches. Nowadays, the open surgical approach, including the minimal invasive one, offers the possibility to implant directly on the damaged myocardial area both scaffolds and hydrogel without limiting surgeons’ choose on different materials.
The percutaneous approach, is surely less-invasive and less risky, but even using a NOGA mapping of the scar tissue, it seems still far from satisfactory results, due to the difficulty to inject high density hydrogels, pre-assembled scaffold or TEVGs. It is now superior to classical surgery only in BRS implantation.
Many efforts are indeed needed to foster surgical community and basic sciences to speed up the adoption of these biomaterials in clinical trials and to adequately validate the effectiveness of this new exciting approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Projections of mortality and causes of death, 2015 and 2030. MORTALITY 2015 and 2030 - BASELINE SCENARIO.
- Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016;388:2743-52. [Crossref] [PubMed]
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629-38. [Crossref] [PubMed]
- Mallone A, Weber B, Hoerstrup SP. Cardiovascular Regenerative Technologies: Update and Future Outlook. Transfus Med Hemother 2016;43:291-6. [Crossref] [PubMed]
- Clifford DM, Fisher SA, Brunskill SJ, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev 2012;2. [PubMed]
- Burridge PW, Keller G, Gold JD, et al. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012;10:16-28. [Crossref] [PubMed]
- Richardson WJ, Clarke SA, Quinn TA, et al. Physiological Implications of Myocardial Scar Structure. Compr Physiol 2015;5:1877-909. [Crossref] [PubMed]
- Holmes JW, Borg TK, Covell JW. Structure and Mechanics of Healing Myocardial Infarcts. Annu Rev Biomed Eng 2005;7:223-53. [Crossref] [PubMed]
- Landmesser U, Wollert KC, Drexler H. Potential novel pharmacological therapies for myocardial remodelling. Cardiovasc Res 2009;81:519-27. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Wei S, Chow LT, Sanderson JE. Effect of carvedilol in comparison with metoprolol on myocardial collagen postinfarction. J Am Coll Cardiol 2000;36:276-81. [Crossref] [PubMed]
- Watanabe R, Ogawa M, Suzuki JI, et al. A comparison between imidapril and ramipril on attenuation of ventricular remodeling after myocardial infarction. J Cardiovasc Pharmacol 2012;59:323-30. [Crossref] [PubMed]
- Dor V, Sabatier M, Di Donato M, et al. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg 1998;116:50-9. [Crossref] [PubMed]
- Batista RJ, Verde J, Nery L, et al. Partial Left Ventriculectomy to Treat End-Stage Heart Disease. Ann Thorac Surg 1997;64:634-8. [Crossref] [PubMed]
- Chaudhry PA, Mishima T, Sharov VG, et al. Passive epicardial containment prevents ventricular remodeling in heart failure. Ann Thorac Surg 2000;70:1275-80. [Crossref] [PubMed]
- Magovern JA. Experimental and Clinical Studies with the Paracor Cardiac Restraint Device. Semin Thorac Cardiovasc Surg 2005;17:364-8. [Crossref] [PubMed]
- Dandel M, Schueler S. Mechanical Circulatory Support as Bridge to Recovery. In: Montalto A, Loforte A, Musumeci F, et al. editors. Mechanical Circulatory Support in End-Stage Heart Failure. Cham: Springer, 2017:131-47.
- Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol 2006;48:907-13. [Crossref] [PubMed]
- Nelson DM, Ma Z, Fujimoto KL, et al. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomater 2011;7:1-15. [Crossref] [PubMed]
- Ma PX. Scaffolds for tissue fabrication. Materials Today 2004;7:30-40. [Crossref]
- Tous E, Purcell B, Ifkovits JL, et al. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res 2011;4:528-42. [Crossref] [PubMed]
- Plotkin M, Vaibavi SR, Rufaihah AJ, et al. The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack. Biomaterials 2014;35:1429-38. [Crossref] [PubMed]
- Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction: a 5-year update. J Am Coll Cardiol 2011;58:2615-29. [Crossref] [PubMed]
- Li XY, Wang T, Jiang XJ, et al. Injectable hydrogel helps bone marrow-derived mononuclear cells restore infarcted myocardium. Cardiology 2010;115:194-9. [Crossref] [PubMed]
- Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 2011;8:607-26. [Crossref] [PubMed]
- Rodell CB, Lee ME, Wang H, et al. Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left-Ventricular Remodeling. Circ Cardiovasc Interv 2016;9. [Crossref] [PubMed]
- Leor J, Tuvia S, Guetta V, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol 2009;54:1014-23. [Crossref] [PubMed]
- Rao SV, Zeymer U, Douglas PS, et al. Bioabsorbable Intracoronary Matrix for Prevention of Ventricular Remodeling After Myocardial Infarction. J Am Coll Cardiol 2016;68:715-23. [Crossref] [PubMed]
- Anker SD, Coats AJ, Cristian G, et al. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur Heart J 2015;36:2297-309. [Crossref] [PubMed]
- Mann DL, Lee RJ, Coats AJ, et al. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur J Heart Fail 2016;18:314-25. [Crossref] [PubMed]
- Frey N, Linke A, Süselbeck T, et al. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ Cardiovasc Interv 2014;7:806-12. [Crossref] [PubMed]
- Singelyn JM, DeQuach JA, Seif-Naraghi SB, et al. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 2009;30:5409-16. [Crossref] [PubMed]
- Singelyn JM, Sundaramurthy P, Johnson TD, et al. Catheter-Deliverable Hydrogel Derived from Decellularized Ventricular Extracellular Matrix Increases Endogenous Cardiomyocytes and Preserves Cardiac Function Post-Myocardial Infarction. J Am Coll Cardiol 2012;59:751-63. [Crossref] [PubMed]
- Seif-Naraghi SB, Singelyn JM, Salvatore MA, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med 2013;5. [Crossref] [PubMed]
- Highley CB, Prestwich GD, Burdick JA. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol 2016;40:35-40. [Crossref] [PubMed]
- Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev 1998;98:1743-54. [Crossref] [PubMed]
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater 2009;5:1-13. [Crossref] [PubMed]
- Krause K, Jaquet K, Schneider C, et al. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: first-in-man study. Heart 2009;95:1145-52. [Crossref] [PubMed]
- D’Amore A, Yoshizumi T, Luketich SK, et al. Bi-layered polyurethane - Extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model. Biomaterials 2016;107:1-14. [Crossref] [PubMed]
- Hashizume R, Hong Y, Takanari K, et al. The effect of polymer degradation time on functional outcomes of temporary elastic patch support in ischemic cardiomyopathy. Biomaterials 2013;34:7353-63. [Crossref] [PubMed]
- Lin YD, Ko MC, Wu ST, et al. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater Sci 2014;2:567-80. [Crossref] [PubMed]
- Shadrin IY, Allen BW, Qian Y, et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun 2017;8:1825. [Crossref] [PubMed]
- Menasché P, Vanneaux V, Hagège A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 2015;36:2011-7. [Crossref] [PubMed]
- Alexander JH, Smith PK. Coronary-Artery Bypass Grafting. N Engl J Med 2016;374:1954-64. [Crossref] [PubMed]
- Slicker K, Lane WG, Oyetayo OO, et al. Daily cardiac catheterization procedural volume and complications at an academic medical center. Cardiovasc Diagn Ther 2016;6:446-52. [Crossref] [PubMed]
- Ju YM, Ahn H, Arenas-Herrera J, et al. Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta Biomater 2017;59:58-67. [Crossref] [PubMed]
- Esquivel CO, Blaisdell FW. Why small caliber vascular grafts fail: a review of clinical and experimental experience and the significance of the interaction of blood at the interface. J Surg Res 1986;41:1-15. [Crossref] [PubMed]
- Greenwald SE, Berry CL. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J Path 2000;190:292-9. [Crossref] [PubMed]
- Benrashid E, McCoy CC, Youngwirth LM, et al. Tissue engineered vascular grafts: Origins, development, and current strategies for clinical application. Methods 2016;99:13-9. [Crossref] [PubMed]
- Li J, Connell S, Shi R. Biomimetic Architectures for Tissue Engineering. In: Mukherjee A. editor. Biomimetics Learning from Nature. InTech, 2010.
- Rieger KA, Birch NP, Schiffman JD. Designing electrospun nanofiber mats to promote wound healing - a review. J Mater Chem B 2013;1:4531-41. [Crossref]
- Lee MH, Kwon BJ, Koo MA, et al. Exovascular application of epigallocatechin-3-O-gallate-releasing electrospun poly(L-lactide glycolic acid) fiber sheets to reduce intimal hyperplasia in injured abdominal aorta. Biomed Mater 2015;10. [PubMed]
- Centola M, Rainer A, Spadaccio C, et al. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2010;2. [Crossref] [PubMed]
- Spadaccio C, Rainer A, Centola M, et al. Heparin-releasing scaffold for stem cells: a differentiating device for vascular aims. Regen Med 2010;5:645-57. [Crossref] [PubMed]
- Zou J, Zhang X, Yang H, et al. Rapamycin-loaded nanoparticles for inhibition of neointimal hyperplasia in experimental vein grafts. Ann Vasc Surg 2011;25:538-46. [Crossref] [PubMed]
- Duncan DR, Chen PY, Patterson JT, et al. TGFβR1 inhibition blocks the formation of stenosis in tissue-engineered vascular grafts. J Am Coll Cardiol 2015;65:512-4. [Crossref] [PubMed]
- Dimitrievska S, Cai C, Weyers A, et al. Click-coated, heparinized, decellularized vascular grafts. Acta biomater 2015;13:177-87. [Crossref] [PubMed]
- Ong CS, Zhou X, Yu Chen, et al. Tissue Engineered Vascular Grafts: Current State of the Field. Expert Rev Med Devices 2017;14:383-92. [Crossref] [PubMed]
- Hajiali H, Shahgasempour S, Naimi-Jamal MR, et al. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int J Nanomedicine 2011;6:2133-41. [Crossref] [PubMed]
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-7. [Crossref] [PubMed]
- Shirota T, He H, Yasui H, et al. Human endothelial progenitor cell-seeded hybrid graft: proliferative and anti-thrombogenic potentials in vitro and fabrication processing. Tissue Eng 2003;9:127-36. [Crossref] [PubMed]
- Mirensky TL, Hibino N, Sawh-Martinez RF, et al. Tissue-engineered vascular grafts: does cell seeding matter? J Pediatr Surg 2010;45:1299-305. [Crossref] [PubMed]
- Matsumura G, Miyagawa-Tomita S, Shin'oka T, et al. First evidence that bone marrow cells contribute to the construction of tissue engineered vascular autografts in vivo. Circulation 2003;108:1729-34. [Crossref] [PubMed]
- Nieponice A, Soletti L, Guan J, et al. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle derived stem cells in a rat model. Tissue Eng Part A 2010;16:1215-23. [Crossref] [PubMed]
- Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, et al. Human adipose stem cells: a potential cell source for cardiovascular tissue engineering. Cells tissues organs 2008;187:263-74. [Crossref] [PubMed]
- Krawiec JT, Liao HT, Kwan LL, et al. Evaluation of the stromal vascular fraction of adipose tissue as the basis for a stem cell-based tissue engineered vascular graft. J Vasc Surg 2017;66:883-90.e1. [Crossref] [PubMed]
- Sundaram S, Echter A, Sivarapatna A, et al. Small-diameter vascular graft engineered using human embryonic stem cell-derived mesenchymal cells. Tissue Eng Part A 2014;20:740-50. [PubMed]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Davoudi P, Assadpour S, Derakhshan MA, et al. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: A promising approach towards stable endothelial lining. Mater Sci Eng C Mater Biol Appl 2017;80:213-21. [Crossref] [PubMed]
- Pashneh-Tala S, MacNeil S, Claeyssens F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng Part B Rev 2015;22:68-100. [PubMed]
- Krawiec JT, Vorp DA. Adult stem cell-based tissue engineered blood vessels: A review. Biomaterials 2012;33:3388-400. [Crossref] [PubMed]
- Hibino N, Duncan DR, Nalbandian A, et al. Evaluation of the use of an induced pluripotent stem cell sheet for the construction of tissue-engineered vascular grafts. J Thorac Cardiovasc Surg 2012;143:696-703. [Crossref] [PubMed]
- Gui L, Dash BC, Luo J, et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 2016;102:120-9. [Crossref] [PubMed]
- Sundaram S, One J, Siewert J, et al. Tissue-engineered vascular grafts created from human induced pluripotent stem cells. Stem Cells Transl Med 2014;3:1535-43. [Crossref] [PubMed]
- Dahl SL, Blum JL, Niklason LE. Bioengineered Vascular Grafts: Can We Make Them Off-the-Shelf? Trends Cardiovasc Med 2011;21:83-9. [Crossref] [PubMed]
- Dahl SL, Kypson AP, Lawson JH, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 2011;3:68ra9. [Crossref] [PubMed]
- Quint C, Arief M, Muto A, et al. Allogeneic human tissue engineered blood vessel. J Vasc Surg 2012;55:790-8. [Crossref] [PubMed]
- Hibino N, Nalbandian A, Devine L, et al. Comparison of Human Bone Marrow Mononuclear Cell Isolation Methods for Creating Tissue-Engineered Vascular Grafts: Novel Filter System Versus Traditional Density Centrifugation Method. Tissue Eng Part C Methods 2011;17:993-8. [Crossref] [PubMed]
- Shin'oka T, Matsumura G, Hibino N, et al. Midterm clinical result of tissue engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 2005;129:1330-8. [Crossref] [PubMed]
- Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissueengineered vascular grafts in humans. J Thorac Cardiovasc Surg 2010;139:431-6, 436.e1-2.
- Bockeria LA, Svanidze O, Kim A, et al. Total cavopulmonary connection with a new bioabsorbable vascular graft: First clinical experience. J Thorac Cardiovasc Surg 2017;153:1542-50. [Crossref] [PubMed]
- McAllister TN, Maruszewski M, Garrido SA, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 2009;373:1440-6. [Crossref] [PubMed]
- Lawson JH, Glickman MH, Ilzecki M, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: Two phase 2 single-arm trials. Lancet 2016;387:2026-34. [Crossref] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT02644941
- Available online: https://clinicaltrials.gov/ct2/show/NCT01305863
- Available online: https://clinicaltrials.gov/ct2/show/NCT01840956
- Available online: https://clinicaltrials.gov/ct2/show/NCT00850252
- Wystrychowski W, McAllister TN, Zagalski K, et al. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg 2014;60:1353-7. [Crossref] [PubMed]
- Kraak RP, Grundeken MJ, Koch KT, et al. Bioresorbable scaffolds for the treatment of coronary artery disease: current status and future perspective. Expert Rev Med Devices 2014;11:467-80. [Crossref] [PubMed]
- Farooq V, Serruys PW, Heo JH, et al. Intracoronary optical coherence tomography and histology of overlapping everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: the potential implications for clinical practice. JACC Cardiovasc Interv 2013;6:523-32. [Crossref] [PubMed]
- Džavík V, Colombo A. The absorb bioresorbable vascular scaffold in coronary bifurcations: insights from bench testing. JACC Cardiovasc Interv 2014;7:81-8. [Crossref] [PubMed]
- Joner M. Chronic vascular response: the view with the microscope. EuroPCR. Paris, France; 2014.
- Foin N, Gutierrez-Chico JL, Nakatani S, et al. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: implications for the management of incomplete stent apposition. Circ Cardiovasc Interv 2014;7:180-9. [Crossref] [PubMed]
- Bourantas CV, Papafaklis MI, Kotsia A, et al. Effect of the endothelial shear stress patterns on neointimal proliferation following drug-eluting bioresorbable vascular scaffold implantation: an optical coherence tomography study. JACC Cardiovasc Interv 2014;7:315-24. [Crossref] [PubMed]
- Fernández-Rodríguez D, Brugaletta S, Otsuki S, et al. Acute ABSORB bioresorbable vascular scaffold thrombosis in ST-segment elevation myocardial infarction: to stent or not to stent? EuroIntervention 2014;10:600-discussion 600. [Crossref] [PubMed]
- Van Geuns RJ. BVS Expand: 6-month results. EuroPCR. Paris, France; 2014.
- Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med 2017;376:2319-28. [Crossref] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT02973529
- Available online: https://clinicaltrials.gov/ct2/show/NCT01845311
- Available online: https://clinicaltrials.gov/ct2/show/NCT01610102
- Available online: https://clinicaltrials.gov/ct2/show/NCT01168830
- Available online: https://clinicaltrials.gov/ct2/show/NCT01960504
- Drakos SG, Kfoury AG, Stehlik J, et al. Bridge to Recovery Understanding the Disconnect Between Clinical and Biological Outcomes. Circulation 2012;126:230-41. [Crossref] [PubMed]