Immediate non-culprit vessel percutaneous coronary intervention (PCI) in patients with acute myocardial infarction and cardiogenic shock: a swinging pendulum
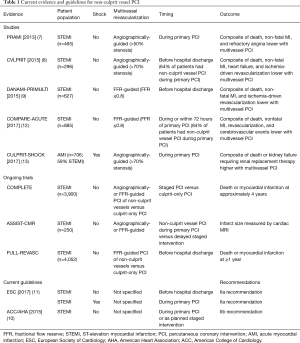
Revascularization of the culprit vessel in patients with acute myocardial infarction (AMI) reduces morbidity and mortality (1,2). Approximately 50% of AMI patients also have significant stenoses in non-infarct related arteries (3). Although multivessel disease is common in patients with AMI and is associated with an increased risk of major adverse cardiovascular events, the management approach to non-infarct related arteries in AMI patients with multivessel disease has not been well established (3,4). Until recently, clinical practice guidelines for the management of ST-elevation myocardial infarction (STEMI) recommended against percutaneous coronary intervention (PCI) of non-culprit vessel stenoses during primary PCI, except for patients in cardiogenic shock (5). Non-culprit vessel PCI in hemodynamically stable patients was indicated at a time separate from primary PCI in patients with symptoms of myocardial ischemia and considered reasonable in patients with intermediate or high-risk findings on non-invasive testing. These recommendations were largely based on observational studies and expert opinion in the absence of robust prospective trial data (6). More recently, a series of small randomized controlled trials have noted safety, and some have demonstrated efficacy with reduced major adverse cardiac events, with routine early PCI of non-culprit vessel disease in hemodynamically stable STEMI patients with multivessel disease (7-9). Based upon these accumulating data, non-culprit vessel PCI at the time of primary PCI or as a planned staged intervention was changed from a class III to a class IIb recommendation in the 2015 American College of Cardiology (ACC)/American Heart Association (AHA) focused update on primary PCI for STEMI, and became a class IIa recommendation in the 2017 European Society of Cardiology (ESC) STEMI guidelines (10,11).
The decision to revascularize and the approach to revascularization (timing, extent, and method) of multivessel disease in patients with AMI is an area of debate and active research. At the current time, there are several ongoing randomized controlled trials examining the effect of non-culprit vessel PCI (COMPLETE NCT01740479; FULL REVASC NCT02862119) and its timing (ASSIST-CMR; NCT01818960) on clinical outcomes and infarct size (Table 1). Non-culprit vessel stenoses diagnosed during coronary angiography for AMI represent vulnerable lesions at future risk of plaque rupture (3). In addition, non-culprit disease in AMI is associated with lower ventricular contractility, larger ischemia burden, and lower blood flow and reperfusion success in the culprit vessel (14). In patients with AMI and cardiogenic shock, non-infarct related artery disease may assume a particular physiological and clinical importance given the delayed recovery of stunned myocardium in the territory of the infarct-related artery. It is therefore conceivable that pre-emptive interventions on non-culprit vessels may help stabilize patients in cardiogenic shock and prevent future adverse cardiovascular events. This conceptual approach to cardiac care made revascularization of non-infarct related artery disease a common practice, performed in approximately 20% of AMI patients with cardiogenic shock (15). However, observational studies of non-culprit vessel revascularization in patients with AMI and cardiogenic shock suggested harm (16). Therefore, a definitive randomized controlled trial was needed.
Full table
In contrast to trials involving hemodynamically stable patients, the results of the recently published CULPRIT-SHOCK trial question the efficacy and safety of immediate complete revascularization of non-infarct related artery disease in AMI patients with cardiogenic shock. In this study, 706 patients with AMI and multivessel disease complicated by cardiogenic shock were randomized to a strategy of immediate multivessel revascularization or culprit-only PCI during the initial procedure (13). The primary outcome was a composite of death or severe renal failure requiring renal replacement therapy. Multivessel disease was defined as stenosis of >70% of vessel diameter in 2 or more major vessels of ≥2 mm in diameter. In the culprit-only arm, lesions in non-infarct related arteries were not to be intervened upon during the initial procedure, but staged revascularization was permitted at the discretion of the treating physician. In the immediate multivessel PCI arm, all major vessels with >70% stenosis were to be revascularized, including attempts at recanalization of chronic total occlusions (CTO) (17). The majority of patients enrolled in the trial presented with STEMI (62%) and had 3-vessel coronary artery disease (63%). Immediate PCI of non-culprit lesions was performed in 90.6% and 12.5% of patients in the immediate and culprit-only PCI arms, respectively. Common reasons for crossover from culprit-only to immediate multivessel PCI included ongoing or worsened hemodynamic instability after culprit-vessel PCI, inability to recanalize the culprit lesion, complications or shifts in plaque after culprit-vessel PCI, and operator preference. In approximately 10% of patients assigned to multivessel PCI, culprit-only PCI was performed due to long procedural times or high contrast load (protocol recommended maximum contrast load of 300 mL), complexity of non-culprit vessel disease, or death before non-culprit vessel disease could be addressed. In an intention-to-treat analysis, immediate multivessel PCI, compared to culprit-only PCI, was associated with an increased risk of the primary outcome (55.4% vs. 45.9%, respectively; P=0.01) (13). This appeared to be driven mainly by an increased mortality rate in the multivessel versus culprit-only PCI (51.6% vs. 43.3%, respectively; P=0.03), with a concomitant trend towards increased renal complications that did not reach statistical significance (16.4% vs. 11.6%, respectively; P=0.07). In addition, there was no difference in time to hemodynamic stabilization, discontinuation of mechanical ventilation, or intensive care unit discharge between treatment arms. Analyses of the per-protocol and as-treated populations yielded similar results, and the treatment effects were consistent across key subgroups (age, type of myocardial infarction, and number of diseased vessels). Renal impairment at baseline or on presentation was not one of the prespecified subgroup analyses in the CULPRIT-SHOCK trial. Patients with AMI and renal impairment prior to or at the time of hospital presentation are a unique subgroup with a higher risk of ischemic, bleeding, and contrast-induced complications (18). Careful examination of CULPRIT-SHOCK outcomes in relation to baseline renal function measures may help elucidate mechanisms of harm with multivessel PCI and further guide clinical practice.
The finding of increased mortality with multivessel revascularization was surprising to some and the exact mechanisms remain to be elucidated. Patients with AMI undergo PCI during a state of heightened platelet activation and aggregation. In CULPRIT-SHOCK, the vast majority of patients were treated with guideline-directed antithrombotic therapy and there were no significant differences in pharmacotherapies between treatment arms. In patients with cardiogenic shock, PCI is often performed during concomitant resuscitation measures, intermittent hemodynamic decompensation, and arrhythmias. Accordingly, one might postulate that PCI performed under these circumstances may be predisposed to peri- or post-procedural complications including cardiac arrest, no reflow, and stent thrombosis. However, when the cause of death was examined in CULPRIT-SHOCK, increased mortality in the multivessel PCI arm did not appear to be attributable to recurrent myocardial infarction, intractable cardiogenic shock, or sudden cardiac death (13). Although ascertaining the exact cause of death is challenging, the rate of death secondary to these etiologies was similar or higher in the culprit-only arm. Instead, increased mortality rate in the multivessel PCI arm appeared to result from brain injury or death from unknown cause. The nature of the association between multivessel PCI and brain injury in CULPRIT-SHOCK patients remains unclear and warrants further examination. Interestingly, while non-culprit vessel PCI increased mortality in CULPRIT-SHOCK, nonculprit vessel PCI in hemodynamically stable patients with STEMI appears to reduce major adverse cardiovascular events. This revascularization paradox where patients at low-to-intermediate risk, but not high risk, benefit from an early and more aggressive intervention has also been noted in post-hoc analyses of trials of pharmacoinvasive strategy for STEMI. In this setting, the treatment effect of early coronary angiography following fibrinolysis was modulated by baseline risk—with clinical benefits in patients at low-to-intermediate risk but not in high-risk patients (19,20).
The results of CULPRIT-SHOCK are pivotal and will likely have a significant impact on clinical practice. The investigators should be commended for their determination to test this clinical hypothesis rigorously in a randomized setting, which has provided valuable information for the management of cardiogenic shock. However, several limitations should be acknowledged when translating their results into real-world practice (Table 2). CULPRIT-SHOCK provides convincing evidence that routine, immediate, and complete revascularization should not be standard practice in most AMI patients with cardiogenic shock (13). However, the results should not deter operators from using clinical judgement as some circumstances may warrant multivessel revascularization. Many operators treating patients in the culprit-only arm of CULPRIT-SHOCK performed multivessel PCI due to lack of hemodynamic improvement, critical non-culprit vessel disease, and clinicians’ preference (13). In addition, although the absolute difference of 8% in mortality rate between the two treatment arms should serve as a cautionary tale, it is noteworthy that multivessel revascularization as defined in CULPRIT-SHOCK was not reflective of current clinical practice. When addressing cardiogenic shock, operators may deem it appropriate to address some, but not all, non-culprit vessel disease at the time of the initial procedure. Complex non-culprit lesions, including CTO, would likely not be routinely intervened on by operators in an acute setting (21). A trial comparing a culprit-only strategy compared to revascularization at the discretion of the treating physician may have yielded different results, as complications arising from protocol-mandated attempts at revascularizing complex lesions may have biased outcomes in favor of a culprit-only strategy. In the CULPRIT-SHOCK design, blinding was not possible due to the nature of the intervention. Despite this, most procedural characteristics appear to be well balanced between treatment arms. However, when examining the use of mechanical circulatory support, there is a trend towards more frequent use of Impella CP percutaneous assist devices in patients in the culprit-only arm and extracorporeal membrane oxygenation in patients in the immediate multivessel PCI arm. Although these differences may represent the play of chance, it is conceivable that the choice and timing of mechanical circulatory support may have been affected by the revascularization strategy and thereby biased the results. Lastly, randomization in CULPRIT-SHOCK was performed without initial consent. While this may improve enrollment and generalizability, the resultant withdrawal of patients due to lack of final consent was problematic as it was unlikely to represent data missing at random.

Full table
The CULPRIT-SHOCK trial provides valuable insight into the current treatment and outcomes in AMI patients presenting with cardiogenic shock. Perhaps the most sobering observation in CULPRIT-SHOCK, the largest revascularization trial for cardiogenic shock to date, is that almost half of patients with AMI complicated by cardiogenic shock died within 30 days of their index event (13). In the SHOCK trial, published almost two decades ago, 30-day mortality in patients undergoing early revascularization for cardiogenic shock was also 47% (2). Although patient demographics have changed over the last 2 decades, large contemporary observational studies show that cardiogenic shock complicating AMI remains associated with high in-hospital mortality (22,23). The CULPRIT-SHOCK results suggest that the path to improved clinical outcomes in AMI patients with cardiogenic shock may not simply lie in routine immediate multivessel revascularization (13). Further research on early interventional and complex critical care of AMI patients with cardiogenic shock is needed. Unfortunately, strategies examined in prospective trials to date have had disappointing results (24-26). At present, temporary mechanical circulatory support as a means of offloading the left ventricle and safeguarding end organ perfusion during the early post-AMI state remains only a theoretical prospect requiring further investigation. The growing use of mechanical circulatory support may facilitate improvements in interventional technologies, patient selection, technical skills, and clinical outcomes creating an ideal substrate for larger and definitive randomized trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Keeley EC, Hillis LD. Primary PCI for myocardial infarction with ST-segment elevation. N Engl J Med 2007;356:47-54. [Crossref] [PubMed]
- Hochman JS, Sleeper LA, Webb JG, et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N Engl J Med 1999;341:625-34. [Crossref] [PubMed]
- Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA 2014;312:2019-27. [Crossref] [PubMed]
- Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 32:2999-3054. [PubMed]
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, O'Gara PT, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-140. [Crossref] [PubMed]
- Hannan EL, Samadashvili Z, Walford G, et al. Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc Interv 2010;3:22-31. [Crossref] [PubMed]
- Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369:1115-23. [Crossref] [PubMed]
- Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol 2015;65:963-72. [Crossref] [PubMed]
- Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet 2015;386:665-71. [Crossref] [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol 2016;67:1235-50. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [PubMed]
- Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med 2017;376:1234-44. [Crossref] [PubMed]
- Thiele H, Akin I, Sandri M, et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med 2017;377:2419-32. [Crossref] [PubMed]
- Cheema AN, Mehta SR, Verma S, et al. Non-infarct related artery revascularization in ST-segment elevation myocardial infarction patients with multivessel disease. Curr Opin Cardiol 2017;32:600-7. [Crossref] [PubMed]
- de Waha S, Jobs A, Eitel I, et al. Multivessel versus culprit lesion only percutaneous coronary intervention in cardiogenic shock complicating acute myocardial infarction: A systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 2018;7:28-37. [PubMed]
- Thiele H, Ohman EM, Desch S, et al. Management of cardiogenic shock. Eur Heart J 2015;36:1223-30. [Crossref] [PubMed]
- Thiele H, Desch S, Piek JJ, et al. Multivessel versus culprit lesion only percutaneous revascularization plus potential staged revascularization in patients with acute myocardial infarction complicated by cardiogenic shock: Design and rationale of CULPRIT-SHOCK trial. Am Heart J 2016;172:160-9. [Crossref] [PubMed]
- Russo JJ, Goodman SG, Cantor WJ, et al. Does renal function affect the efficacy or safety of a pharmacoinvasive strategy in patients with ST-elevation myocardial infarction? A meta-analysis. Am Heart J 2017;193:46-54. [Crossref] [PubMed]
- Yan AT, Yan RT, Cantor WJ, et al. Relationship between risk stratification at admission and treatment effects of early invasive management following fibrinolysis: insights from the Trial of Routine ANgioplasty and Stenting After Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER-AMI). Eur Heart J 2011;32:1994-2002. [Crossref] [PubMed]
- Bagai A, Tan M, Di Mario C, et al. Routine invasive management early after fibrinolysis: relationship between baseline risk and treatment effects in a pooled patient-level analysis of 7 randomized controlled trials. Am Heart J 2014;168:757-65. [Crossref] [PubMed]
- Henriques JP, Hoebers LP, Ramunddal T, et al. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients With STEMI: The EXPLORE Trial. J Am Coll Cardiol 2016;68:1622-32. [Crossref] [PubMed]
- Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal Trends and Outcomes of Patients Undergoing Percutaneous Coronary Interventions for Cardiogenic Shock in the Setting of Acute Myocardial Infarction: A Report From the CathPCI Registry. JACC Cardiovasc Interv 2016;9:341-51. [Crossref] [PubMed]
- Kolte D, Khera S, Aronow WS, et al. Trends in Incidence, Management, and Outcomes of Cardiogenic Shock Complicating ST-Elevation Myocardial Infarction in the United States. J Am Heart Assoc 2014;3:e000590. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 2017;38:3523-31. [Crossref] [PubMed]
- Alexander JH, Reynolds HR, Stebbins AL, et al. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA 2007;297:1657-66. [Crossref] [PubMed]


