An initial exploration for comprehensive assessment of IgG4-related lung disease: analyses on the cases enrolled from a systematic review
Introduction
Since the first report of lung involvement in immunoglobulin G4-related disease (IgG4-RD) in 2004, increasing attention has been paid to IgG4-related lung disease (IgG4-RLD) (1). IgG4-RLD is a systemic disease characterized by IgG4 positive plasma cell infiltration, storiform fibrosis, and obliterative phlebitis with or without elevated serum IgG4 concentration. The interstitium, mediastinum, airways and pleura are organs involved in IgG4-RLD (2).
As many cases of IgG4-RD have been reported, concern about the diagnostic criteria has arisen. Comprehensive diagnostic criteria for IgG4-RD were released by the Japan College of Rheumatology in 2011 (Japan criteria); meanwhile, a consensus statement on the pathology of IgG4-RD was reached in Boston, United States (Boston criteria) (3,4). The two diagnostic systems have similar features, such as an emphasis on histopathology; otherwise, the diagnostic criteria from Japan consider the affected organs, elevated serum IgG4 concentrations and histopathology as equally important. By contrast, the Boston criteria are based on histopathology alone. The divergences between the two systems are in the number of IgG4+ plasma cells/high-power field (HPF), diagnostic efficacy of the serum IgG4 concentration and ratio of IgG4+/IgG+ cells. The existence of these two diagnostic systems could be confusing for clinicians. Does the diagnosis of reported IgG4-RLD in the literature agree with the two diagnostic systems? A study comparing the Boston criteria and global assessment with respect to IgG4-RD was recently published; however, detailed information about the global assessment was not provided (5). Here, we tentatively explored a scoring system for comprehensively assessing the diagnosis of IgG4-RLD, which was supposed to merge the merits of the two existing diagnostic systems.
Methods
Literature search
We searched the PubMed, Web of Science and Cochrane Library databases for relevant articles. To include as many papers related to IgG4-RLD as possible, we searched using the following terms: ((IgG4-related) OR Immunoglobulin G4-related) AND ((lung disease) OR pulmonary disease). There were no limits on the ethnicity or region; all papers were published in English and the search was updated as of February 2017. Studies included in this article met the following criteria: published in English; discussed IgG4-RLD; obtained pathological tissue from the lung; and full text available. For cases from the same hospital, we chose the most recent report.
Patient inclusion and data extraction
Articles contained in our study include case reports that can provide detailed information on the patients and original articles from which specific data cannot be extracted. Data from case reports include the author, year, region, gender, age, clinical features, lesion location, the method for obtaining specimens, pathological features, the ratio of IgG4+/IgG+ plasma cells, IgG4+/HPF count, serum IgG4/IgG concentration (mg/dL) and IgG4-RLD therapy. Data from original articles include the author, year, region, sample size, gender, age, respiratory allergic history, serum IgG4/IgG concentration and therapy. We summarized all data.
If the specific count of IgG4+ plasma cells /HPF was not provided, we gave an approximate count of IG4+ plasma cells according to the description of IgG4+ cells and the provided histological images of Haematoxylin-Eosin staining.
Diagnostic criteria
We used the Boston criteria (3) and Japan criteria (4) to evaluate all patients. The histological groups of the Boston criteria are referred to as Category 1—histologically highly suggestive of IgG4-RLD; Category 2—probable histological features of IgG4-RLD; and Category 3—insufficient histopathological features of IgG4-RLD. The clinicopathological groups for the Japan criteria are referred to as Group Definite, Group Probable, Group Possible and Group Not.
According to the weight of each diagnostic item in the Boston and Japan criteria, we tentatively developed a new scoring system, the comprehensive assessment (Table 1). We divided all patients into four groups according to the following scores (using increment of 4 levels): C1—more than 9.5; C2—7.5, 8, 8.5, 9; C3—5.5, 6, 6.5, 7; and C4—less than 5. C1 could be diagnosed as IgG4-RLD, C2 is highly suggestive of IgG4-RLD, C3 is possible IgG4-RLD, and C4 is not IgG4-RLD.

Full table
Statistical analysis
A Kruskal-Wallis non-parametric test and Mann-Whitney U test was adopted for multiple and individual variables, respectively. Comparison of the IgG4+/IgG+ ratios, number of pathological features and IgG4+ plasma cell count/HPF was made among different categories in the Boston criteria, different groups in the Japan criteria and different groups in the comprehensive assessment. A kappa Consistency test and a McNemar matching chi-square test were used to test the consistency (Kappa ≥0.75 indicated good agreement, 0.75> Kappa ≥0.4 indicated general consistency and Kappa <0.4 indicated poor consistency) and difference. Statistical analysis was performed using GraphPad prism v7 and statistical package for social science (SPSS) version 19.0. A P value less than 0.05 was considered statistically significant.
Results
Literature results
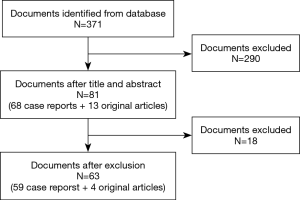
A total of 371 possible articles were found through electronic and manual searches. After browsing the titles and abstracts, 290 articles were eliminated. According to the inclusion criteria, 18 articles were excluded. A total of 63 articles, including 59 case reports and four original articles, were enrolled (Figure 1).
Case reviews before using diagnostic criteria
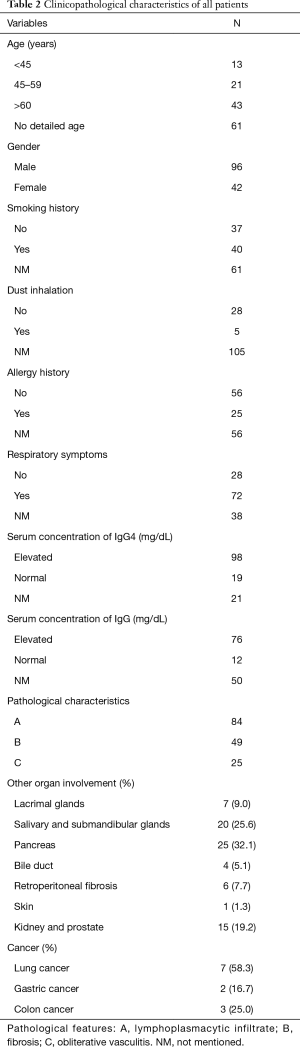
We conducted a brief review to assess the current status of IgG4-RLD. A total of 138 patients were included in our study, including 77 from case reports and the remainder from original articles (see Table S1, which summarizes patients included from original articles). Among all the published articles included in our study, 35 articles are from Japan, 7 are from the USA, 5 are from China, 4 are from Korea, 3 are from the UK, 2 are from Australia, and the remainder are from Canada, Holland, Germany, India, Israel, Greece and Poland. The clinicopathological characteristics are listed in Table 2. There were twice as many male patients as female patients. The number of smokers was approximately equal to that of non-smokers, and few patients have a history of dust exposure. Neither a history of respiratory allergy nor a respiratory symptom was specific to the disease. The involvement of extra-pulmonary organs also varied, and secretory glands were the most common. Approximately 10% of patients had cancer with the discovery of cancer prior to or following the diagnosis of IgG4-RLD. Accompanying diseases were diverse. For histopathological features, dense lymphoplasmacytic infiltration was the most common, which was followed by fibrosis; obliterative phlebitis was often absent.
Full table
Full table
Case reviews after using diagnostic criteria
As we could not obtain detailed information for the patients from original articles, we chose the patients from case reports for further analysis. Each case was evaluated by the Boston criteria, Japan criteria and comprehensive assessment (Table 3). The number of cases in each group was as follows: Boston criteria—22 in Category 1, 14 in Category 2, and 41 in Category 3; Japan criteria—24 in Group Definite, 7 in Group Probable, 37 in Group Possible, and 9 in Group Not; and comprehensive assessment—26 in C1, 15 in C2, 19 in C3, and 17 in C4.
Full table
Comparison of the number of pathological features, IgG4+/IgG+ ratio and IgG4+ plasma cell count/HPF for each group in each criterium
For the Boston criteria, both the ratio of IgG4+/IgG+ and the IgG4+ cell count/HPF were significantly higher in Category 1 compared to Category 3 (both: P<0.01) and in Category 2 compared to Category 3 (ratio: P<0.01; IgG4+cell count: P<0.001). The comparison of the number of pathological features showed a certain degree of distinction (Figure 2).
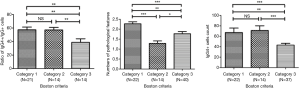
In the comparison for the Japan criteria, neither the ratio of IgG4+/IgG+ nor the IgG4+ cell count/HPF was significantly different among the groups. However, the distinction in the numbers of pathological features was relatively good (Figure 3).

The comparison for comprehensive assessment was somewhat similar to the Boston criteria except for the count of the IgG4+ plasma cells/HPF, in which the degree of difference was even more pronounced (Figure 4).

Consistency between different criteria
Given the sample of each group in the Japan and Boston criteria, we merged the Group Possible and Group Not in the Japan criteria to make the number of levels in both the Boston criteria and Japan criteria match. The Kappa value is 0.482 (P<0.001) between the Boston and Japan criteria (see Table S2, which demonstrates the cross tabulation between the Boston and Japan criteria).
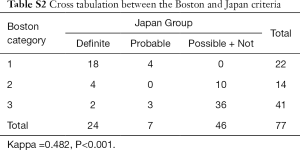
Full table
In the consistency test between Japan and comprehensive criteria, the Kappa value was 0.645 (P<0.001, see Table S3, which demonstrates the cross tabulation between the Japan and comprehensive criteria).
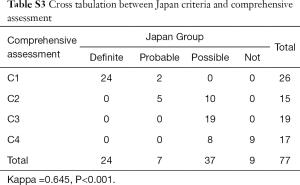
Full table
Given the sample of each group in the comprehensive assessment and Boston criteria, we merged Groups C3 and C4 in the comprehensive assessment to make the number of levels in both the Boston criteria and comprehensive assessment match. The Kappa value is 0.811 (P<0.001, Table 4). The difference between the Boston criteria and comprehensive assessment was significant (P=0.011, McNemar matching test).
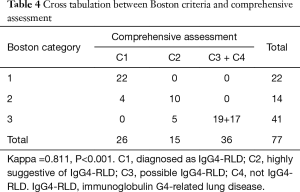
Full table
Discussion
We reviewed 138 cases of IgG4-RLD in the literature and focused on 77 patients with detailed information to evaluate the efficiency of the two existing diagnostic systems. The Boston criteria showed good distinction among its three categories, while the Japan criteria had some blurring between categories. Given the inconsistency between the two existing systems, the current study sought to establish a new comprehensive assessment based on the weight of each diagnostic item in the Boston and Japan Criteria. The proposed scoring system of the comprehensive assessment combined the advantages of both existing criteria, making better use of the clinicopathological information, which was thought to be more efficient than the existing criteria.
Our analysis showed that the classification in the Japan criteria was not pathologically reasonable. Japan criteria seemed to be better than Boston and comprehensive criteria in the distinction in the numbers of pathological features, however, no good distinction in ratio of IgG4+/IgG+ cells and count of IgG4+ plasma cells/HPF was found in Japan criteria. Actually, the diagnosis of IgG4-RLD doesn’t merely rely on the three pathological features. The key advantage of the Japan criteria is the application of the serum IgG4 concentration, which is thought to be linked to the disease. In contrast, the difference in the pathological items among three categories in the Boston criteria was relatively significant. However, the Boston criteria are pathological diagnostic criteria with strict threshold values for the number of pathological features, count of IgG4+ plasma cells and ratio of IgG4+/IgG+. Some potential IgG4-RLD patients might be excluded by the Boston criteria due to insufficient diagnostic information or not meeting a certain threshold. For example, a patient with two pathological features, a 67% IgG4+/IgG+ ratio and 30 IgG4+ plasma cells/HPF [Table 3, reference 8 (8)], could not be diagnosed as IgG4-RLD by the Boston criteria. Similarly, a patient with lymphoplasmacytic infiltration, a 68% IgG4+/IgG+ ratio, 128 IgG4+ plasma cells/HPF, and IgG4 >135 mg/dL [Table 3, reference 11 (11)] was diagnosed as possible IgG4-RLD by the Japan criteria due to the lack of fibrosis. However, should such patients be diagnosed as IgG4-RLD or just suspicious of IgG4-RLD? These two patients were in Group C2 for the comprehensive assessment, which is highly suggestive of IgG4-RLD.
Three primary pathological characteristics in diagnosing IgG4-RLD are dense lymphoplasmacytic infiltration, fibrosis, and obliterative phlebitis. However, fibrosis or obliterative phlebitis can be absent or indistinctive in the lung (65). We found similar results that dense lymphoplasmacytic infiltration was the most common, which was followed by fibrosis; obliterative phlebitis was often absent (Table 2). Therefore, lymphoplasmacytic infiltration scored 2 points, fibrosis scored 1.5 points, and obliterative phlebitis scored 0.5 points in our scoring system. The ratio of IgG4+/IgG+ plasma cells is another powerful diagnostic item in diagnosing IgG4-RD, and the suggested cut-off value is >40% in any organ (66-68). The cut-off value of >40% is adopted as a diagnostic item in both criteria, but it is a mandatory item in the Boston criteria. For this reason, we adopted four points for the ratio of IgG4+/IgG >40% in our scoring system. The appropriate cut-off value for the IgG4+ plasma cell/HPF varies for different organs. The count of IgG4+ plasma cells may be elevated in inflammatory conditions, lymphoma and malignancies (3). The count >50/HPF in surgical specimens or > 20/HPF in biopsy specimens, adopted in the Boston Criteria, is usually highly specific, although the count of IgG4+ plasma cells/HPF alone is not specific (69,70). It should be noted that the count of IgG4+ plasma cells/HPF alone could not be used as a powerful pathological feature in diagnosing IgG4-RD (71). Therefore, the count of IgG4+ cells/HPF did not weigh heavily in our comprehensive assessment compared to the ratio of IgG4+/IgG. From the result of the IgG4 serum concentration, 84% patients had an elevated serum IgG4 concentration (Table 2, 98/117). The cut-off value for the serum IgG4 concentration is 135 mg/dL, which was decided based on receiver operating characteristic (ROC) curves in AIP patients (72,73). An elevated IgG4 serum concentration can also be seen in atopic dermatitis, pemphigus, asthma, and multicentric Castleman’s disease (4). We thought that an elevated serum IgG4 concentration could be a prompt screening test for IgG4-RLD. However, because an elevated serum IgG4 concentration was unable to be interpreted as a high IgG4 cell count or ratio in pulmonary lesions, we gave 0.5 points to the serum IgG4 concentration in the scoring system. Although the scoring system was arbitrary, the weight of each diagnostic item was based on the literature review.
The Boston criteria just cover the histopathological features; however, the diagnosis of IgG4-RLD requires a clinical picture context beyond appropriate histopathological features (3). Based on statistical analysis, the ratio of IgG4+/IgG+ plasma cells was significantly higher in C1 and C2 than in C3 and C4 in the comprehensive assessment, which is similar to the Boston criteria, where the ratio of IgG4+/IgG+ plasma cells was significantly higher in Categories 1 and 2 than Category 3. Additionally, we found a more obvious difference between C3 and C1+C2, but an unnoticeable difference between C3 and C4 in the IgG4+ plasma cell count/HPF, which was similar to the Boston criteria where the count of IgG4+ plasma cells was significantly higher in Categories 1 and 2 than Category 3. Therefore, one of the significant points in the comprehensive assessment is the existence of Group C3, which possibly includes those cases excluded by the Boston criteria due to unmet histopathological features. C3 is similar to Group Possible in the Japan criteria. The diagnosis of IgG4-RLD could still possibly be made in C3 when other diagnoses are excluded. From Table 4, we could find that four patients in Boston Category 2 could be diagnosed as C1 in comprehensive criteria, five patients in Boston Category 3 could be diagnosed as C2 in comprehensive criteria and 19 patients in Boston Category 3 could be diagnosed as C3 in comprehensive criteria. The consistency test showed good agreement between the Boston criteria and comprehensive assessment, unlike that between the Boston criteria and Japan criteria, in which two cases were in Boston Category 3 but Japan Group Definite (Table S2). Additionally, the comprehensive assessment differs from the Boston and Japan criteria in that it could not only make better use of clinicopathological information but also consider the weight of each diagnostic item. This is another significant advantage of the comprehensive assessment.
In clinical practice, we have two options for acquiring tissue, surgical biopsy or needle biopsy. In our study, needle biopsy was adopted in 20 cases. Surgical biopsy is probably better than needle biopsy. Sometimes needle biopsy cannot capture the complete histological information for the biopsy sample (3,74). Needle biopsy supplies limited tissue and obliterative phlebitis may not be identified in a specimen of small size (74). Sometimes, peritumoural tissue contains abundant IgG4+ plasma cells. If peripheral tissue outside of the malignancy is acquired by needle biopsy, a misdiagnosis of IgG4-RLD is made. Then, could the diagnosis of IgG4-RLD be made without biopsy as in IgG4-related autoimmune pancreatitis (IgG4-AIP)? The answer is probably “no”. IgG4-RD is similar to malignant tumours and diseases, such as Sjogren’s syndrome, Castleman’s disease, Wegener’s granulomatosis, and sarcoidosis. IgG4-RLD could be misdiagnosed as lung cancer, nonspecific interstitial pneumonia, lymphoproliferative disorder, sarcoidosis, or tuberculosis (31,46,47,74). Therefore, histopathological examination is essential to diagnosing IgG4-RLD. Video-assisted thoracoscopic surgery is an option for obtaining a specimen for an accurate diagnosis.
There are some limitations in our study. First, the incidence of IgG4-RLD is low and there is no gold standard for diagnosing IgG4-RLD. That is why we used systematic review to enroll patients. Second, some patients included in this study lacked specific quantitative information for the diagnosis. Additionally, heterogeneity exists in the method for counting IgG4+ plasma cells/HPF, the magnification of the microscope, the immunostaining of IgG4 and IgG and the detection of serum IgG4 concentration in the evaluated studies. Nonetheless, the thorough literature review and analysis should be very helpful for development of more meaningful criteria.
Conclusions
The Boston criteria and Japan criteria have relatively poor consistency. The comprehensive assessment has good agreement with the Boston criteria, but it can detect those cases in Boston Category 3 that could still be diagnosed as IgG4-RLD. Considering the weight of the diagnostic items, the scoring system of the comprehensive assessment is a tentative exploration and should be improved with further experience in diagnosing IgG4-RLD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Duvic C, Desrame J, Leveque C, et al. Retroperitoneal fibrosis, sclerosing pancreatitis and bronchiolitis obliterans with organizing pneumonia. Nephrol Dial Transplant 2004;19:2397-9. [Crossref] [PubMed]
- Campbell SN, Rubio E, Loschner AL. Clinical review of pulmonary manifestations of IgG4-related disease. Ann Am Thorac Soc 2014;11:1466-75. [Crossref] [PubMed]
- Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181-92. [Crossref] [PubMed]
- Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012;22:21-30. [Crossref] [PubMed]
- Bateman AC, Culver EL. IgG4-related disease-experience of 100 consecutive cases from a specialist centre. Histopathology 2017;70:798-813. [Crossref] [PubMed]
- Kobayashi H, Shimokawaji T, Kanoh S, et al. IgG4-positive pulmonary disease. J Thorac Imaging 2007;22:360-2. [Crossref] [PubMed]
- Takato H, Yasui M, Ichikawa Y, et al. Nonspecific interstitial pneumonia with abundant IgG4-positive cells infiltration, which was thought as pulmonary involvement of IgG4-related autoimmune disease. Intern Med 2008;47:291-4. [Crossref] [PubMed]
- Tsuboi H, Inokuma S, Setoguchi K, et al. Inflammatory pseudotumors in multiple organs associated with elevated serum IgG4 level: recovery by only a small replacement dose of steroid. Intern Med 2008;47:1139-42. [Crossref] [PubMed]
- Yamashita K, Haga H, Kobashi Y, et al. Lung involvement in IgG4-related lymphoplasmacytic vasculitis and interstitial fibrosis: report of 3 cases and review of the literature. Am J Surg Pathol 2008;32:1620-6. [Crossref] [PubMed]
- Shrestha B, Sekiguchi H, Colby TV, et al. Distinctive pulmonary histopathology with increased IgG4-positive plasma cells in patients with autoimmune pancreatitis: report of 6 and 12 cases with similar histopathology. Am J Surg Pathol 2009;33:1450-62. [Crossref] [PubMed]
- Ikari J, Kojima M, Tomita K, et al. A case of IgG4-related lung disease associated with multicentric Castleman's disease and lung cancer. Intern Med 2010;49:1287-91. [Crossref] [PubMed]
- Miyashita T, Yoshioka K, Nakamura T, et al. A case of lymphomatoid granulomatosis-like lung lesions with abundant infiltrating IgG4-positive plasma cells whose serum IgG4 levels became high following the start of corticosteroid therapy. Intern Med 2010;49:2007-11. [Crossref] [PubMed]
- Fujiu K, Sakuma H, Miyamoto H, et al. Immunoglobulin G4-related inflammatory pseudotumor of the lung. Gen Thorac Cardiovasc Surg 2010;58:144-8. [Crossref] [PubMed]
- Toyoshima M, Chida K, Kono M, et al. IgG4-related Lung Disease in a Worker Occupationally Exposed to Asbestos. Internal Medicine 2010;49:1175-8. [Crossref] [PubMed]
- Dias OM, Kawassaki Ade M, Haga H, et al. Immunoglobulin G4-related systemic sclerosing disease in a patient with sclerosing cholangitis, inflammatory pseudotumors of the lung and multiple radiological patterns: a case report. Clinics (Sao Paulo) 2011;66:1983-6. [Crossref] [PubMed]
- Nishikawa G, Nakamura K, Yamada Y, et al. Inflammatory pseudotumors of the kidney and the lung presenting as immunoglobulin G4-related disease: a case report. J Med Case Rep 2011;5:480. [Crossref] [PubMed]
- Yamamoto H, Suzuki T, Yasuo M, et al. IgG4-Related Pleural Disease Diagnosed by a Re-Evaluation of Chronic Bilateral Pleuritis in a Patient Who Experienced Occasional Acute Left Bacterial Pleuritis. Internal Medicine 2011;50:893-7. [Crossref] [PubMed]
- Chapman EM, Gown A, Mazziotta R, et al. Pulmonary hyalinizing granuloma with associated elevation in serum and tissue IgG4 occurring in a patient with a history of sarcoidosis. Am J Surg Pathol 2012;36:774-8. [Crossref] [PubMed]
- Odaka M, Mori S, Asano H, et al. Thoracoscopic resection for a pulmonary nodule with the infiltrate of IgG4-positive plasma cells. Asian J Endosc Surg 2012;5:176-8. [Crossref] [PubMed]
- Sekiguchi H, Horie R, Utz JP, et al. IgG4-related systemic disease presenting with lung entrapment and constrictive pericarditis. Chest 2012;142:781-3. [Crossref] [PubMed]
- Sugino K, Gocho K, Ishida F, et al. Acquired hemophilia A associated with IgG4-related lung disease in a patient with autoimmune pancreatitis. Intern Med 2012;51:3151-4. [Crossref] [PubMed]
- Tanaka K, Nagata K, Tomii K, et al. A case of isolated IgG4-related interstitial pneumonia: a new consideration for the cause of idiopathic nonspecific interstitial pneumonia. Chest 2012;142:228-30. [Crossref] [PubMed]
- Umeda M, Fujikawa K, Origuchi T, et al. A case of IgG4-related pulmonary disease with rapid improvement. Mod Rheumatol 2012;22:919-23. [Crossref] [PubMed]
- de Jong WK, Kluin PM, Groen HM. Overlapping immunoglobulin G4-related disease and Rosai-Dorfman disease mimicking lung cancer. Eur Respir Rev 2012;21:365-7. [Crossref] [PubMed]
- Hui P, Mattman A, Wilcox PG, et al. Immunoglobulin G4-related lung disease: a disease with many different faces. Can Respir J 2013;20:335-8. [Crossref] [PubMed]
- Ito K, Imafuku S, Hamaguchi Y, et al. Case report of anti-transcription intermediary factor-1-gamma/alpha antibody-positive dermatomyositis associated with gastric cancer and immunoglobulin G4-positive pulmonary inflammatory pseudotumor. J Dermatol 2013;40:567-9. [Crossref] [PubMed]
- Kitada M, Matuda Y, Hayashi S, et al. IgG4-related lung disease showing high standardized uptake values on FDG-PET: report of two cases. J Cardiothorac Surg 2013;8:160. [Crossref] [PubMed]
- Pifferi M, Di Cicco M, Bush A, et al. Uncommon pulmonary presentation of IgG4-related disease in a 15-year-old boy. Chest 2013;144:669-71. [Crossref] [PubMed]
- Suzuki H, Watanabe M, Ara T, et al. Immunoglobulin G4-related lung disease accompanied by organizing pneumonia. Intern Med 2013;52:2105-11. [Crossref] [PubMed]
- Wibmer T, Kropf-Sanchen C, Rudiger S, et al. Isolated IgG4-related interstitial lung disease: unusual histological and radiological features of a pathologically proven case. Multidiscip Respir Med 2013;8:22. [Crossref] [PubMed]
- Ahn JH, Hong SI, Cho DH, et al. A Case of IgG4-Related Lung Disease Presenting as Interstitial Lung Disease. Tuberc Respir Dis (Seoul) 2014;77:85-9. [Crossref] [PubMed]
- Bajema KL, Daniel JC, Hussain S, et al. IgG4-related pulmonary and salivary disease associated with pulmonary tuberculosis. Ann Am Thorac Soc 2014;11:1165-7. [Crossref] [PubMed]
- Choi IH, Jang SH, Lee S, et al. A Case Report of IgG4-Related Disease Clinically Mimicking Pleural Mesothelioma. Tuberc Respir Dis (Seoul) 2014;76:42-5. [Crossref] [PubMed]
- Choi JH, Sim JK, Oh JY, et al. A Case of IgG4-Related Disease Presenting as Massive Pleural Effusion and Thrombophlebitis. Tuberc Respir Dis (Seoul) 2014;76:179-83. [Crossref] [PubMed]
- Inoue T, Hayama M, Kobayashi S, et al. Lung cancer complicated with IgG4-related disease of the lung. Ann Thorac Cardiovasc Surg 2014;20 Suppl:474-7. [Crossref] [PubMed]
- Ishida M, Miyamura T, Sato S, et al. Pulmonary arterial hypertension associated with IgG4-related disease. Intern Med 2014;53:493-7. [Crossref] [PubMed]
- Ishimoto H, Yatera K, Shimabukuro I, et al. Case of immunoglobulin G4 (IgG4)-related disease diagnosed by transbronchial lung biopsy and endobronchial ultrasound-guided transbronchial needle aspiration. J UOEH 2014;36:237-42. [Crossref] [PubMed]
- Omokawa A, Komatsuda A, Hirokawa M, et al. Membranous nephropathy with monoclonal IgG4 deposits and associated IgG4-related lung disease. Clin Kidney J 2014;7:475-8. [Crossref] [PubMed]
- Onishi Y, Kawamura T, Kagami R, et al. IgG4-related lung disease with organizing pneumonia effectively treated with azathioprine. Intern Med 2014;53:2701-4. [Crossref] [PubMed]
- Sun X, Peng M, Hou X, et al. Refractory IgG4-related lung disease with constitutional symptoms and severe inflammation. Am J Respir Crit Care Med 2014;189:374-5. [Crossref] [PubMed]
- Zhou J, Li X, Zeng Q. IgG4-related lung disease with atypical CT imaging: a case report. J Thorac Dis 2014;6:E276-80. [PubMed]
- Hazzard C, Wolf AS, Beasley MB, et al. Benign imitation of malignancy: avoiding resection in immunoglobulin g4-related lung disease. Ann Thorac Surg 2014;98:1465-7. [Crossref] [PubMed]
- Fukihara J, Kondoh Y, Taniguchi H, et al. Pulmonary hypertension associated with obliterative phlebitis in IgG4-related lung disease. Eur Respir J 2015;45:842-5. [Crossref] [PubMed]
- Jinnur PK, Yi ES, Ryu JH, et al. Cavitating Lung Disease: A Novel Presentation of IgG4-Related Disease. Am J Case Rep 2015;16:478-82. [Crossref] [PubMed]
- Krause ML, Yi ES, Warrington KJ. Pulmonary IgG4-related disease and colon adenocarcinoma: possible paraneoplastic syndrome. Int J Rheum Dis 2017;20:654-6. [Crossref] [PubMed]
- Tan H, Li H, Hu Y, et al. A case of solely lung-involved IgG4-related disease mimicking tuberculosis. Heart Lung 2015;44:161-4. [Crossref] [PubMed]
- Wang J, Zeng Y, Gu Y, et al. IgG4-related lung disease manifested as pneumonia in puerperium: a case report. Int J Clin Exp Pathol 2015;8:3312-5. [PubMed]
- Saeki S, Horio Y, Hirosako S, et al. Elevated serum IgG4 levels in two cases of paragonimiasis. Respirol Case Rep 2015;3:92-4. [Crossref] [PubMed]
- Singh RK, Isaac TJ, Thangakunam B, et al. Isolated pulmonary manifestation of IgG4 disease with response to steroids and relapse: A rare case report. Lung India 2015;32:659-61. [Crossref] [PubMed]
- Baltaxe E, Shulimzon T, Lieberman S, et al. IgG4-Related Lung Disease - Three Untreated Cases with a Benign Outcome. Arch Bronconeumol 2016;52:e1-3. [PubMed]
- Grewal K, Cohen P, Kwon JS, et al. IgG4-related disease presenting as a lung mass and weight loss: Case report and review of the literature. Respir Med Case Rep 2015;17:27-9. [Crossref] [PubMed]
- Ikeda S, Sekine A, Baba T, et al. Abundant immunoglobulin (Ig)G4-positive plasma cells in interstitial pneumonia without extrathoracic lesions of IgG4-related disease: is this finding specific to IgG4-related lung disease? Histopathology 2017;70:242-52. [Crossref] [PubMed]
- Kang MK, Cho Y, Han M, et al. IgG4-Related Lung Disease without Elevation of Serum IgG4 Level: A Case Report. Tuberc Respir Dis (Seoul) 2016;79:184-7. [Crossref] [PubMed]
- Kotetsu Y, Ikegame S, Takebe-Akazawa K, et al. A case of IgG4-related lung disease complicated by asymptomatic chronic Epstein-Barr virus infection. Clin Respir J 2017;11:1012-7. [Crossref] [PubMed]
- Noguchi S, Yatera K, Jinbo M, et al. IgG4-related Lung Disease Associated with Autoimmune Hemolytic Anemia: A Case Report and a Literature Review. Intern Med 2016;55:2469-74. [Crossref] [PubMed]
- Onishi Y, Nakahara Y, Hirano K, et al. IgG4-related disease in asbestos-related pleural disease. Respirol Case Rep 2015;4:22-4. [Crossref] [PubMed]
- Patel M, Kumar B, Diep ML, et al. IgG4 Related Lung Disease. Can Respir J 2016;2016:1409281. [PubMed]
- Schneider F, Veraldi KL, Levesque MC, et al. IgG4-Related Lung Disease Associated with Usual Interstitial Pneumonia. Open Rheumatol J 2016;10:33-8. [Crossref] [PubMed]
- Skopouli FN, Panayotopoulos P, Moutsopoulos HM. IgG4-Related Nodular Lung Lesion. Arthritis Rheumatol 2017;69:438. [Crossref] [PubMed]
- Stamatopoulos A, Patrini D, Koletsis E, et al. IgG4 related lung disease extending to the thoracic vertebrae. Respir Med Case Rep 2016;19:162-5. [Crossref] [PubMed]
- Szczawinska-Poplonyk A, Wojsyk-Banaszak I, Jonczyk-Potoczna K, et al. Pulmonary manifestation of immunoglobulin G4-related disease in a 7-year-old immunodeficient boy with Epstein-Barr virus infection: a case report. Ital J Pediatr 2016;42:58. [Crossref] [PubMed]
- Tashiro H, Takahashi K, Nakamura T, et al. Coexistence of lung cancer and immunoglobulin G4-related lung disease in a nodule: a case report. J Med Case Rep 2016;10:113. [Crossref] [PubMed]
- Touge H, Tomita K, Yamasaki A, et al. A case of proteinase 3 anti-neutrophil cytoplasmic antibody (PR3-ANCA) positive/IgG4-related lung disease. Respir Med Case Rep 2017;20:92-4. [Crossref] [PubMed]
- Okubo T, Oyamada Y, Kawada M, et al. Immunoglobulin G4-related disease presenting as a pulmonary nodule with an irregular margin. Respirol Case Rep 2016;5:e00208. [Crossref] [PubMed]
- Zen Y, Inoue D, Kitao A, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol 2009;33:1886-93. [Crossref] [PubMed]
- Cheuk W, Yuen HK, Chu SY, et al. Lymphadenopathy of IgG4-related sclerosing disease. Am J Surg Pathol 2008;32:671-81. [Crossref] [PubMed]
- Cheuk W, Chan JK. IgG4-related sclerosing disease: a critical appraisal of an evolving clinicopathologic entity. Adv Anat Pathol 2010;17:303-32. [Crossref] [PubMed]
- Sato Y, Kojima M, Takata K, et al. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman's disease. Mod Pathol 2009;22:589-99. [Crossref] [PubMed]
- Dhall D, Suriawinata AA, Tang LH, et al. Use of immunohistochemistry for IgG4 in the distinction of autoimmune pancreatitis from peritumoral pancreatitis. Hum Pathol 2010;41:643-52. [Crossref] [PubMed]
- Zhang L, Notohara K, Levy MJ, et al. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol 2007;20:23-8. [Crossref] [PubMed]
- Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol 2011;64:237-43. [Crossref] [PubMed]
- Okazaki K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol 2006;41:626-31. [Crossref] [PubMed]
- Aithal GP, Breslin NP, Gumustop B. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;345:147-8. [Crossref] [PubMed]
- Inoue D, Zen Y, Abo H, et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology 2009;251:260-70. [Crossref] [PubMed]
- Matsui S, Hebisawa A, Sakai F, et al. Immunoglobulin G4-related lung disease: clinicoradiological and pathological features. Respirology 2013;18:480-7. [Crossref] [PubMed]
- Shirai Y, Tamura Y, Yasuoka H, et al. IgG4-related disease in pulmonary arterial hypertension on long-term epoprostenol treatment. Eur Respir J 2014;43:1516-9. [Crossref] [PubMed]
- Sun X, Liu H, Feng R, et al. Biopsy-proven IgG4-related lung disease. BMC Pulm Med 2016;16:20. [Crossref] [PubMed]