Percutaneous occluder device closure through femoral vein guidance by transthoracic echocardiography in adult atrial septal defect patients
Introduction
Atrial septal defect (ASD) is one of the most common congenital heart defects (1). Three techniques are currently available for treatment of a simple ASD: intracardiac repair under direct vision, medical interventional occlusion, and surgical small-incision occlusion. Interventional occlusion is seldom performed for ASDs with a diameter of >35 mm, while for surgical small-incision occlusion, this limitation can be extended to 42 mm based on our clinical experience. Transcatheter treatment is the current gold standard, surgery requires the patient to be anesthetized and a small incision needs to be placed in the chest wall, and is usually only considered in cases with contra-indications to percutaneous delivery. But, while interventional occlusion involves no surgical incision, this method traditionally requires irradiation with fluoroscopy to allow imaging guidance (1).
As newer imaging methods have developed, such as real-time three-dimensional imaging, alongside technical modifications, and newer concepts for hemodynamic evaluation interventional occlusion has become popular in the pediatric population (2), and now is becoming of interest in adults (3). These improved imaging methods provide high quality imaging allowing accurate anatomical measurement of the maximum defect size, the morphology of the surrounding rim, and allows evaluation of the relationship between the device and septal rim (2). In this study we used transthoracic echocardiography guidance which has been shown to be safe, effective, and easy during transcatheter ASD closure (4). The novelty of this study is that there was also no need for fluoroscopic guidance during the ASD closure.
In the present study of patients with simple, centrally located ASDs, we combined the characteristics of interventional and surgical repair using an ASD occluder through femoral vein puncture guided by transthoracic ultrasonography without radiation under local anesthesia. The information we provide should assist clinicians who select a similar method for treatment of simple ASDs.
Methods
Patient selection
From May 2014 to July 2016, 14 patients underwent percutaneous occluder device closure through femoral vein guiding by transthoracic echography in the Department of Cardiac Surgery, Beijing Anzhen Hospital, Capital Medical University. To be eligible for inclusion in the case series the criteria were: (I) aged 18 and over; (II) a secundum ASD (central type) and left-to-right shunt; (III) the diameter of the defect <38 mm; (IV) grade I cardiac function; (V) no or mild tricuspid regurgitation, no right ventricular volume overloading, no moderate or severe pulmonary hypertension, no other combined cardiac malformations, and no other system dysfunction. This research was submitted to the Ethics Committee of Capital Medical University, and they issued a declaration of its legality, approval number ID was 2014012X. Informed consents were waived by the Ethics Committee.
Operative procedure
The patients were placed in the supine position on the operating table (Figure 1). They were sedated, but remained awake for the procedure. The groin area was surgically prepared and draped, and the anterior chest wall was exposed for ultrasound examination (Figure 2). After ultrasonic measurement of the ASD diameter, an appropriate occluder was selected, and a SteerEase sheath delivery kit (Lifetech Scientific, Shenzhen, China) with an appropriate aperture was selected. The length of the guide wire was measured, and a point was marked on the fourth rib at the right edge of the sternum. After intravenous administration of heparin (1 mg/kg of body weight), femoral vein puncture was performed, and the guide wire was inserted into the right atrium (Figure 3). It may or may not pass through the ASD at this point (the ultrasound imaging was unclear compared with radiation because the guide wire was too thin). After the skin incision had been enlarged with a dilator, the delivery sheath was inserted and pre-filled with heparin saline to exhaust the air within it. After the delivery sheath had reached the right atrium, it could be clearly seen on ultrasound imaging. The camber on the tip of the delivery sheath was used to adjust the direction of the sheath, enabling it to pass across the ASD. The size of the occluder (Heart R; Lifetech Scientific) was 6 to 8 mm larger than the largest diameter of the ASD. The front of the septal occluder was partially released in a spherical shape. The occluder showed hyperechogenicity and a small amount of bubble overflow under ultrasound imaging. Under ultrasound guidance, the occluder was adjusted to an appropriate position, and its front and rear were released (Figure 4). The residual shunt of the ASD and the mitral, tricuspid, and aortic valve activities were then observed by ultrasound. Electrocardiography was performed to identify any conduction blocks; if none were present, the delivery cable was fully released (Figures 5-8). Upon completion of the operation, protamine was administered to neutralize the heparin, the femoral vein was compressed for 10 minutes, and the patient was sent to the general ward for electrocardiographic monitoring. Oral aspirin was administered postoperatively.
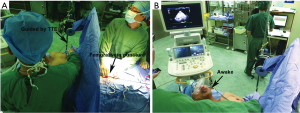


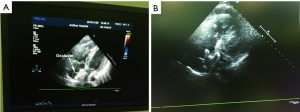




Patients were followed up with ultrasonic cardiogram for 2 years after operation.
Results
Fourteen patients (3 males and 11 females) aged 31.5 years (19–64 years) with a secundum ASD (central type) and left-to-right shunt are presented in this case series. The diameter of the defect ranged from 9 to 32 mm (Table 1).
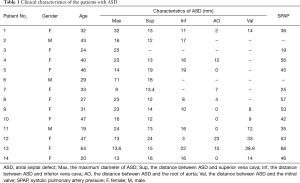
Full table
All fourteen patients underwent successful operations using septal occluders with diameters of 16 to 36 mm. A small amount of bubble overflow occurred upon release of the septal occluder, but no gas embolisms developed. The mean operative duration was 18.7±22.5 (range, 12–56) minutes. No intraoperative conversions to other anesthetic methods or different types of incisions occurred, and no patients developed thrombogenesis or embolism.
The median hospital stay was 2.3±0.5 days (1–3 days). No patients developed intraoperative puncture site bleeding, cardiac perforation, pericardial tamponade, arrhythmia, or other complications. Postoperative ultrasound examination showed no residual shunt, valvular regurgitation, oppression of the aortic valve, or poor mitral valve leaflet activity. No patients developed postoperative bleeding, hemolysis, or occluder displacement or loss. The 2-year postoperative follow-up showed no residual shunt, valvular regurgitation, arrhythmia, endocarditis, or other complications.
Discussion
We present a small case series of patients who underwent novel fluoroscopy-free percutaneous occluder device closure of ASD through the femoral vein, using guidance by transthoracic echocardiography under local anesthesia.
The therapeutic study of ASD occlusion began in 1974. In 1976, King et al. (11) first attempted to occlude ASDs in adult patients by transcatheter delivery of a double-umbrella patch occluder. Although they succeeded, the diameter of the delivery system for their delivery patch was 23 French (69 mm), and clinical application was therefore extremely difficult for central type secundum ASDs with a diameter of ≤20 mm. In 1976 (12), the first-generation Rashkind double-umbrella occluder was created, and occlusion treatment was successfully performed in patients with ASD. In 1989, Lock et al. (13) designed a double-umbrella occluder called the Clamshell occluder for treatment of ASDs, but clinical trials revealed a high residual shunt rate. In 1990, Sideris (14) applied a button-type double-disk occlusion device for treatment of ASDs. This device had a positive patch on the left atrial side and a negative patch on the right atrial side. Although hundreds of ASDs were occluded in adults and infants, the popularization and application of this device failed because of the operation complexity and high residual shunt rate. A new generation of nitinol woven occluders was developed by the manufacturer of Amplatzer (St. Jude Medical, St. Paul, MN, USA) in 1997 and significantly improved the safety and success rate of the interventional treatment of congenital heart disease. Thus, medical interventional occlusion has been most frequently applied to the treatment of simple congenital heart disease (15).
Treatment of ASDs by surgical occlusion began in the last century. In 1997, Amin et al. (16) was the first to successfully perform surgical occlusion of a ventricular septal defect in an infant patient with a muscular ventricular septal defect without cardiopulmonary bypass. In 2003, Amin (17) successfully performed transventricular perimembranous septal defect occlusion in animal experiments. In the same year, Bacha applied perimembranous septal defect occlusion to clinical practice. In 2005, Diab et al. (18) used a hybrid method for the intraoperative transventricular closure of multiple muscular ventricular septal defects. In 2007, Diab et al. (19) reported that the Amplatzer occluder was applied for transatrial occlusion of ASDs. In China in 2003, Kang (20) performed the first repair of ASDs in a large group of patients using transthoracic small-incision occlusion without cardiopulmonary bypass. In the early period, surgical occlusion was mostly performed for ventricular septal defects that would have been difficult to repair with medical intervention. For ASDs, transthoracic puncture small-incision right atrial occlusion has the following advantages over interventional occlusion. First, there is no exposure to radiation; second, it is still applicable for some larger defects (diameter of >35 mm); and third, the hospital costs are relatively low. Therefore, this technique remains the first choice for some patients. Of course, surgical transthoracic occlusion still requires endotracheal intubation, general anesthesia, transesophageal ultrasound guidance, and a 2-cm incision in the chest wall.
Upon completion of hundreds of transthoracic small-incision and ASD occlusions (21), we have accumulated a large amount of experience in transesophageal echocardiography and occlusion techniques for this procedure. Direct comparison with our previous results (21), shows the advantages of this current technique compared to catheter occlusion, the current gold standard, there was no radiographic visualization of fluoroscopy and this method had a shorter operation time (18.7±22.5 vs. 65.4±20.8 min) and shorter length of hospital stay (2.3±0.5 vs. 9.7±4.5). If we compare this novel method with our previous experience of minimally invasive occlusion it shows advantages of no general anesthetic, no requirement for intubation, no need for incision, shorter operation time (18.7±22.5 vs. 61.9±9.0 min) and shorter hospital stay (2.3±0.5 vs. 10.5±3.4). The success rate was also higher with the novel method than either of the previous methods (21), but as the sample size is quite small, further studies are needed to compare the success of these different techniques. Although we tried to apply the medical intervention method and equipment, we have completely adopted transthoracic echocardiography-guided occlusion. For our first patient who underwent this surgical technique, we still applied endotracheal intubation, general anesthesia, and transesophageal ultrasound guidance and prepared and draped the chest wall, allowing us to convert to the chest wall small-incision pathway in case of failure. The second patient did not undergo endotracheal intubation or general anesthesia, but instead underwent occlusion under transesophageal ultrasound guidance. Although the occlusion was successful, this patient experienced more severe pain than the first patient. After consultation with the ultrasonographers and anesthesiologists of our institution, we applied the occlusion method without anesthesia, using transthoracic ultrasound guidance and the transfemoral vein puncture pathway; we successfully completed six surgeries using this technique. Considering the unknown technical difficulty and risks, in the early period we chose young patients with simple, medium-sized ASDs with edges of >5 mm. Later, with improvement in the technical procedure, we also performed this procedure in older patients with ASDs that lacked certain edges, and all procedures were successful. The fifth case took a relatively long time to complete because the patient had a larger defect. The maximum diameter was 38 mm, and the aorta end edge was 0 mm. The front of the occluder did not easily become stuck after it was released, and it fell back to the right atrium. After several attempts, the “waist” of the occluder “held” the aortic root, and the procedure was finally successful. We could efficiently proceed with the occlusion because the patient was in the surgery room and well prepared for intravenous inhalational anesthesia and conversion to small-incision occlusion or even immediate intracardiac repair under direct vision with extracorporeal circulation, if necessary.
As previously described, the guide wire on the ultrasound image is not obvious, so it must be marked well in advance to ensure that its tip reaches the right atrium. The guide wire does not necessarily pass across the ASD and may slightly release the front of the septal occluder. Thus, a small, ball-shaped hyperechoic structure is present at the front end of the delivery sheath on the ultrasound image, making it easy to locate. This is the main difference between performing this procedure under ultrasound versus under radiation. Our experience with how to locate the occluder under ultrasound comes from the hundreds of small-incision transthoracic occlusion operations we have performed before.
Many reports have described the similarities in the complications and long-term prognosis after atrial septal Amplatzer occlusion, interventional occlusion, and surgical occlusion (22-24); therefore, we have not discussed these details in the present report. Our patients were followed up for 2 years, and no significant adverse events occurred, which is consistent with other studies.
Limitations
As a retrospective case series, this study has some limitations, including its small sample size. There was no comparison with more traditional methods to evaluate whether the safety and outcomes were preferable. There was also no comparison with fluoroscopy guided ASD occlusion, this should be done in the future to compare patient outcomes.
Conclusions
In conclusion, this study suggests that, as a minimally invasive, safe, simple, and new surgical technique, use of an ASD occluder through femoral vein puncture guided by transthoracic ultrasonography without radiation under local anesthesia can be applied to more adult patients. We hope this information assists other clinicians considering undertaking a similar procedure for the treatment of simple ASDs in adults.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This research was submitted to the Ethics Committee of Capital Medical University, and they issued a declaration of its legality, approval number ID was 2014012X. Informed consents were waived by the Ethics Committee.
References
- Vasquez AF, Lasala JM. Atrial septal defect closure. Cardiol Clin 2013;31:385-400. [Crossref] [PubMed]
- Ammar RI, Hegazy RA. Transcatheter closure of secundum ASD using Occlutech Figulla-N device in symptomatic children younger than 2 years of age. J Invasive Cardiol 2013;25:76-9. [PubMed]
- Akagi T. Current concept of transcatheter closure of atrial septal defect in adults. J Cardiol 2015;65:17-25. [Crossref] [PubMed]
- Acar P, Massabuau P, Elbaz M. Real-time 3D transoesophageal echocardiography for guiding Amplatzer septal occluder device deployment in an adult patient with atrial septal defect. Eur J Echocardiogr 2008;9:822-3. [PubMed]
- Jia Y, Meng X, Li Y, et al. The patient was sedated but remained awake for the procedure. Asvide 2018;5:371. Available online: http://www.asvide.com/article/view/23833
- Jia Y, Meng X, Li Y, et al. The femoral vein was punctured, and the guide wire was inserted into the right atrium. Asvide 2018;5:372. Available online: http://www.asvide.com/article/view/23834
- Jia Y, Meng X, Li Y, et al. The occluder was installed and soaked with heparin saline to exhaust the air within it. Asvide 2018;5:373. Available online: http://www.asvide.com/article/view/23836
- Jia Y, Meng X, Li Y, et al. The delivery sheath was inserted along the guide wire and passed across the atrial septal defect. Asvide 2018;5:374. Available online: http://www.asvide.com/article/view/23838
- Jia Y, Meng X, Li Y, et al. The occluder was placed under ultrasound guidance and released after satisfactory ultrasound examination. Asvide 2018;5:375. Available online: http://www.asvide.com/article/view/238340
- Jia Y, Meng X, Li Y, et al. The residual shunt of the atrial septal defect and the mitral, tricuspid, and aortic valve activities were observed by ultrasound. Asvide 2018;5:376. Available online: http://www.asvide.com/article/view/238341
- King TD, Thompson SL, Steiner C, et al. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976;235:2506-9. [Crossref] [PubMed]
- Rashkind WJ. Transcatheter treatment of congenital heart disease. Circulation 1983;67:711-6. [Crossref] [PubMed]
- Lock JE, Rome JJ, Davis R, et al. Transcatheter closure of atrial septal defects. Experimental studies. Circulation 1989;79:1091-9. [Crossref] [PubMed]
- Sideris EB, Sideris SE, Fowlkes JP, et al. Transvenous atrial septal defect occlusion in piglets with a "buttoned" double-disk device. Circulation 1990;81:312-8. [Crossref] [PubMed]
- Zabal-Cerdeira C, Garcia-Montes JA, Sandoval-Jones JP, et al. Percutaneous closure of atrial septal defects with the Amplatzer(R) device: 15 years of experience. Arch Cardiol Mex 2014;84:250-5. [Crossref] [PubMed]
- Amin Z, Gu X, Berry JM, et al. Perventricular [correction of Periventricular] closure of ventricular septal defects without cardiopulmonary bypass. Ann Thorac Surg 1999;68:149-53; discussion 153-4. [Crossref] [PubMed]
- Amin Z, Danford DA, Lof J, et al. Intraoperative device closure of perimembranous ventricular septal defects without cardiopulmonary bypass: preliminary results with the perventricular technique. J Thorac Cardiovasc Surg 2004;127:234-41. [Crossref] [PubMed]
- Diab KA, Hijazi ZM, Cao QL, et al. A truly hybrid approach to perventricular closure of multiple muscular ventricular septal defects. J Thorac Cardiovasc Surg 2005;130:892-3. [Crossref] [PubMed]
- Diab KA, Cao QL, Bacha EA, et al. Device closure of atrial septal defects with the Amplatzer septal occluder: safety and outcome in infants. J Thorac Cardiovasc Surg 2007;134:960-6. [Crossref] [PubMed]
- Kang YF, Yu SQ, Cai ZJ. Minimal invasive surgical closure of secundum atrial septal defect without cardiopulmonary bypass. J Fourth MiI Med Univ 2003;24:2.
- Zeng W, Meng X, Zhang C, et al. Comparison study of mini-invasive surgery, transcatheter closure and open-heart surgery for treatment of Secundum Atrial Septal Defect. Journal of Cardiovascular and Pulmonary Diseases 2009;28:14-7.
- Du ZD, Hijazi ZM, Kleinman CS, et al. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 2002;39:1836-44. [Crossref] [PubMed]
- Fischer G, Stieh J, Uebing A, et al. Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients. Heart 2003;89:199-204. [Crossref] [PubMed]
- Chen Q, Cao H, Zhang GC, et al. Safety and feasibility of intra-operative device closure of atrial septal defect with transthoracic minimal invasion. Eur J Cardiothorac Surg 2012;41:121-5. [PubMed]


