Values of aortic dissection detection risk score combined with ascending aorta diameter >40 mm for the early identification of type A acute aortic dissection
Introduction
Acute aortic dissection (AAD) is defined as the disruption of the media layer of the aorta with bleeding within and along the wall of the aorta resulting in the separation of the layers of the aorta (1), including type A acute aortic dissection (A-AAD), with the involvement of the ascending aorta, and type B acute aortic dissection (B-AAD), without involving the ascending aorta. The morbidity rate of AAD is only 6 cases per 100,000 person-years (2), whereas its prognosis appears to be worse with 19.9% dying in the hospital. In particular, the overall in-hospital mortality rate of A-AAD is up to 24.4%, which is significantly more than that of B-AAD (10.7%) (3). Moreover, its mortality rate increases 1–2% per hour after symptom onset (4). In particular, A-AAD is more likely to have a concomitant acute myocardial infarction (AMI) (5). However, the missed diagnosis of A-AAD in patients with AMI secondary to A-AAD might increase the mortality rate of A-AAD. Therefore, the early diagnosis and treatment of A-AAD might play a role in attenuating the mortality rate of AAD.
To detect AAD early and rapidly in patients presenting with acute chest pain, the American College of Cardiology (ACC) and American Heart Association (AHA) guidelines for the diagnosis and management of patients with thoracic aortic disease proposed the aortic dissection detection (ADD) risk score system based on predisposing conditions, pain features, and clinical examination. Although the risk score system has a high sensitivity of 95.7% for the detection of AAD (6), its specificity is much lower (39.8%) (7). In recent years, numerous researchers have attempted to seek several novel methods for the early identification of AAD (6-14). For instance, the combined use of the ADD risk score and D-dimer was studied as part of the current diagnostic work-up of patients with AAD (13,14). However, D-dimer has a high sensitivity (96.6%) but a lower specificity (46.6%) for the diagnosis of AAD (12). As one convenient, rapid, flexible imaging technique, the transthoracic echocardiography (TTE) has a high specificity but a relatively lower sensitivity for the detection of AAD. More importantly, there are lots of direct signs and indirect signs of the TTE that have been used for the diagnosis of A-AAD. Additionally, several signs of the TTE are only picked up by doctors who specialize in echocardiography, consequently affecting its clinical application. Nevertheless, approximately 90% of patients with A-AAD demonstrate an ascending aorta diameter >40 mm (9,15-18), and the ascending aorta diameter can be obtained from the TTE by emergency physicians easily, immediately and accurately, which also makes its application possible for the early identification of A-AAD.
However, to date, studies about the values of the combined use of the risk score and an ascending aorta diameter >40 mm for the detection of A-AAD are still lacking. Thus, first of all, the diagnostic accuracy of the ADD risk score, combined with an ascending aorta diameter >40 mm for A-AAD, was assessed. Then, this novel model was used for the early identification of A-AAD in patients with AMI secondary to A-AAD.
Methods
Patients
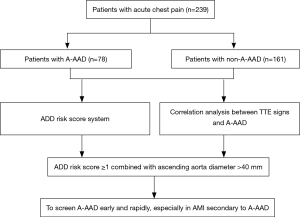
A total of 239 patients presenting with acute chest pain on admission to Shanghai General Hospital, School of Medicine, Shanghai Jiao Tong University between July 2010 and December 2016 were enrolled in our study, and all the following inclusion criteria had to be satisfied: (I) the time from symptom onset to admission was all within 14 days; (II) they were all required to receive the TTE in the emergency department or intensive care unit and (III) the diagnosis of AAD was confirmed by computed tomography angiography (CTA), according to the 2014 European Society of Cardiology (ESC) guidelines on the diagnosis and treatment of aortic diseases (19). The flowchart of this study is shown in Figure 1. We retrospectively collected all the patients’ clinical characteristics, including risk factors, clinical manifestations, diagnosis, treatments and outcomes. This study was approved by the Ethics Committee of Shanghai General Hospital (No. 2017KY233).
ADD risk score system
The ADD risk score system, provided by the 2010 ACC/AHA guidelines for the diagnosis and management of patients with thoracic aortic disease (1), covers the following three sections:
The high-risk pain features included chest, back, or abdominal pain described as the following: abrupt in oneself; severe in intensity; and a ripping/tearing/sharp or stabbing quality.
The high-risk examination features encompassed evidence of a perfusion deficit (pulse deficit; systolic blood pressure differential; focal neurologic deficit), a new murmur of aortic insufficiency, and hypotension or a shock state.
The high-risk conditions consisted of Marfan syndrome, connective tissue disease, family history aortic disease, known aortic valves disease, recent aortic manipulation, and known thoracic aortic aneurysm.
The guidelines noted that patients with acute chest pain could have a score of 1 as long as they satisfied any marker of the above three sections. Therefore, the patients with acute chest pain were divided into three groups as follow: low risk if the score was 0; intermediate risk if the score was 1; and high risk if the score was 2 or 3.
TTE
In the emergency department or intensive care unit, the structure of the heart was routinely scanned by a Philips SONOS 5500 color Doppler ultrasonic diagnostic apparatus with a 1.8–3.6 MHz probe. Then, the aorta was visualized utilizing the following views: the left parasternal; high left parasternal; apical or subcostal. The aortic flow filling and distribution was observed dynamically. As described previously (1), the left (and sometimes right) parasternal view of the TTE was best for visualizing the ascending aorta.
Statistical analysis
SPSS for Windows version 19.0 software (SPSS Inc., Chicago, Illinois, USA) was used for all the statistical analysis. The continuous variables are presented as the mean ± standard deviation (SD) and were compared by an independent-samples t test. The categorical variables are presented as frequencies and percentages and were compared by a chi square test. The receiver operating characteristic (ROC) curve was used to analyze the diagnostic performance of the aortic root diameter and the ascending aorta diameter for A-AAD. Values of P <0.05 were considered statistically significant.
Results
Baseline characteristics
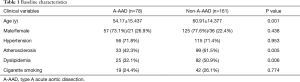
The baseline characteristics are summarized in Table 1. This study consisted of 239 patients, including 78 patients with A-AAD and 161 patients with non-A-AAD. There was no significant difference among sex between the patients with A-AAD and patients with non-A-AAD. The patients with non-A-AAD were older than the patients with A-AAD (60.91±14.377 versus 54.17±15.437, P=0.001). The percentage of patients with A-AAD who had a history of atherosclerosis was lower than the patients with patients with non-A-AAD (42.3% versus 61.5%, P=0.005). More patients with non-A-AAD presented with a history of dyslipidemia than the patients with A-AAD (50.9% versus 32.1%, P=0.006).
Full table
ADD risk score
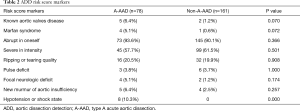
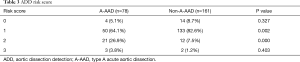
The risk score markers of the patients with A-AAD and the patients with non-A-AAD are shown in Table 2. The patients with A-AAD were more likely to present with hypotension or a shock state compared with the patients with non-A-AAD (10.3% versus 0, P=0.000). The risk scores are presented in Tables 3,4. The proportion of the patients with A-AAD who scored 1 was lower than the patients with non-A-AAD (64.1% versus 82.6%, P=0.002). The percentage of the patients with A-AAD who scored 2 was higher than the patients with non-A-AAD (26.9% versus 7.5%, P=0.000). Moreover, 30.8% of the patients with A-AAD showed a risk score ≥2, whereas a risk score ≥2 only existed in 8.7% of the patients with non-A-AAD. This difference was statistically significant (P=0.000).
Full table
Full table

Full table
The diagnostic accuracy of the risk score for the detection of A-AAD is shown in Table 5. A risk score ≥1 had an excellent sensitivity of 94.9% and a fair negative predictive value (NPV) of 77.8%, with a poor specificity of 8.7% and a positive predictive value (PPV) of 33.5% for the diagnosis of A-AAD. A risk score ≥2 had an excellent specificity of 91.3% and a fair NPV of 73.1%, whereas it had a lower sensitivity of 30.8% and a PPV of 63.2%.

Full table
Direct and indirect signs of the TTE for the diagnosis of A-AAD
The direct signs (ascending aorta dilation, aorta root dilation, and intimal flap) and indirect signs (aortic regurgitation, pericardial effusion, and left ventricular wall motion abnormality) of the TTE are presented in Table 6. An ascending aorta diameter of >40 mm existed in 89.7% of the patients with A-AAD, whereas only 13.7% of the patients with non-A-AAD showed an ascending aorta diameter >40 mm. This difference was statistically significant (P=0.000). There were no differences among the aortic regurgitation and the left ventricular wall motion abnormality between the patients with A-AAD and the patients with non-A-AAD. The intimal flap existed in 26.9% of the patients with A-AAD, whereas only 4.3% of the patients with non-A-AAD showed the intimal flap. This difference was statistically significant (P=0.000). The patients with A-AAD were more likely to present with pericardial effusion than patients with non-A-AAD (11.5% versus 3.1%, P=0.021).
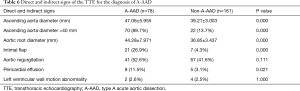
Full table
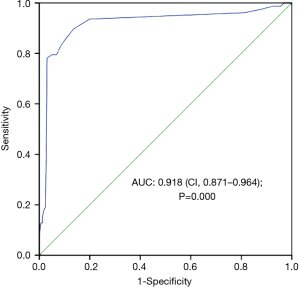
The ascending aorta diameter of the patients with non-A-AAD was significantly lower than that of the patients with A-AAD (39.21±3.003 versus 47.06±5.955 mm, P=0.000). Additionally, the aortic root diameter of the patients with A-AAD was significantly higher than that of the patients with non-A-AAD (44.28±7.971 versus 36.85±3.437 mm, P=0.000). In the ROC curve showing the diagnostic performance of the aortic root diameter for A-AAD, its area under the curve (AUC) was 0.871 and its 95% confidence interval (CI) was 0.814–0.928 (P=0.000). However, as shown in Figure 2, the AUC area was 0.918 and the 95% CI was 0.871–0.964 (P=0.000). When the ascending aorta diameter was 40.50 mm, which could be regarded as the optimal cut-off value for the diagnosis of A-AAD, the Youden index (YI) had a maximum value of 0.76.
Combined use of the ADD risk score and an ascending aorta diameter >40 mm
As shown in Table 4, the proportion of patients with A-AAD who demonstrated a risk score ≥1 and an ascending aorta diameter >40 mm was higher than the patients with non-A-AAD (84.6% versus 12.4%, P=0.000). The percentage of patients with non-A-AAD who demonstrated a risk score ≥2 and an ascending aorta diameter >40 mm was lower than the patients with A-AAD (1.9% versus 24.4%, P=0.000).
The diagnostic accuracy of the risk score combined with an ascending aorta diameter >40 mm for A-AAD is presented in Table 5. A risk score ≥1, combined with an ascending aorta diameter >40 mm, had a sensitivity, a specificity, a PPV, and an NPV of 84.6%, 87.6%, 76.7%, and 92.2% for the diagnosis of A-AAD, respectively. The combined use of a risk score ≥2 and an ascending aorta diameter >40 mm had an excellent specificity of 98.1% and a PPV of 86.4%, a fair NPV of 72.8%, and a poor sensitivity of 24.4% for the detection of A-AAD.
Combined use of the ADD risk score and an ascending aorta diameter >40 mm decreased the omission diagnostic rate of A-AAD in patients with AMI secondary to A-AAD
In our center, the omission diagnostic rate of A-AAD in 27 patients with AMI secondary to A-AAD was up to 33.3% (9/27). As shown in Table 7, although the omission diagnostic rate of A-AAD was increased to 63.0% by risk score ≥2 combined with an ascending aorta diameter >40 mm, the combined use of a risk score ≥1 and an ascending aorta diameter >40 mm significantly decreased the omission diagnostic rate of A-AAD in patients with AMI secondary to A-AAD. The omission diagnostic rate of A-AAD was only 7.4%.

Full table
Discussion
To date, although the D-dimer, the TTE, and the ADD risk score have been applied for the early identification of AAD, an effective tool for the early detection of A-AAD is still missing. In the present study, we combined the ADD risk score with an ascending aorta diameter >40 mm to construct a novel model for the early identification of A-AAD and evaluated the diagnostic performance of this model for A-AAD. The findings indicated that the combined use of an ADD risk score ≥1 and an ascending aorta diameter >40 mm was highly indicative of A-AAD in patients presenting with acute chest pain, especially in patients with AMI secondary to A-AAD, which urgently needs CTA or magnetic resonance imaging (MRI) to confirm the diagnosis of A-AAD.
We chose an ascending aorta diameter > 40 mm as one index of this novel model based on the following reasons: on the one hand, the left (and sometimes right) parasternal view of the TTE was best for visualizing the ascending aorta and the aortic root (1), whereas other signs of the TTE, such as the intimal flap, the pericardial effusion and the left ventricular wall motion abnormalities, were difficult for doctors who did not specialize in echocardiography to discover. Therefore, emergency physicians can be easily trained to obtain the ascending aorta diameter from the TTE immediately and accurately. On the other hand, based on the ROC curve analysis, we concluded that the ascending aorta diameter had a greater value than the aortic root diameter for the detection of A-AAD. Additionally, previous studies suggest that approximately 90% of patients with A-AAD demonstrate an ascending aorta diameter >40 mm (9,15-18). In the present study, an ascending aorta diameter >40 mm existed in 89.7% of the patients with A-AAD, which was consistent with previous studies. More importantly, the ROC curve analysis demonstrated that an ascending aorta diameter equal to 40.50 mm could be regarded as the optimal cut-off value of for the diagnosis of A-AAD. Thus, we combined the ADD risk score with an ascending aorta diameter >40 mm to further investigate their values in the early identification of A-AAD.
To the best of our knowledge, this was the first study to propose the values of the ADD risk score, combined with an ascending aortic diameter >40 mm, for the early identification of A-AAD. Nazerian et al. found that a risk score ≥2 and direct transthoracic focus cardiac ultrasound signs, including the intimal flap and the intramural hematoma, could increase the specificity (81% to 98%) and the PPV (31% to 75%) for the detection of A-AAD compared with a risk score ≥2, whereas the sensitivity was attenuated from 40% to 24% and the NPV (86% to 86%) remained unchanged (11). In the present study, although the combined use of a risk score ≥2 and an ascending aorta diameter >40 mm increased the specificity (91.3% to 98.1%) and the PPV (63.2% to 86.4%) for the diagnosis of AAD compared with a risk score ≥2, the sensitivity and the NPV were only reduced by 6.4% and 0.3%, respectively. A risk score ≥1, combined with an ascending aorta >40 mm, increased the specificity (8.7% to 87.6%, P=0.000), the PPV (33.5% to 76.7%), and the NPV (77.8% to 92.2%) for the detection of A-AAD compared with a risk score ≥1, but the sensitivity (94.9% to 84.6%, P=0.035) decreased. In conclusion, although it slightly decreased the sensitivity of the risk score, the combined use of a risk score ≥1 and an ascending aorta diameter >40 mm significantly increased the specificity of the risk score for the diagnosis of A-AAD, which could also be regarded as an optimal model for the early identification of A-AAD in patients presenting with acute chest pain.
Approximately 7% of patients with AAD have concomitant AMI (1). However, in patients with AMI secondary to AAD, the omission diagnostic rate of AAD is as high as 30% (20). In the present study, the omission diagnostic rate of A-AAD was up to 33.3%. This may be because the AMI can be confirmed by clinical manifestations, the electrocardiogram, and cardiac troponin T or I level, while the diagnosis of AAD only depends on imaging modalities (CTA or MRI). That is to say, it is hardly possible to diagnose AAD at the time of first medical contact (FMC). In particular, the guideline for patients with ST-segment elevation myocardial infarction (STEMI) is that antiplatelet strategy should be given at the time of FMC, and patients should be sent to the cardiac catheterization laboratory for the interventional diagnosis and treatment as soon as possible (21). Even the latest guideline for STEMI recommends clinicians to initiate this treatment within 10 min from STEMI diagnosis if the reperfusion strategy is fibrinolysis (22). These drive clinicians to neglect the diagnosis of A-AAD in patients with AMI secondary to A-AAD. However, for patients with AMI, the missed diagnosis of A-AAD could delay the treatment of A-AAD and aggravate the dissection (the antiplatelet therapy, the fibrinolysis strategy, and the cardiac catheterization). In the present study, if this novel model (ADD risk score ≥1 combined with ascending aorta diameter >40 mm) is applied for the early identification of A-AAD, the omission diagnostic rate of A-AAD might significantly decrease in patients with AMI secondary to A-AAD.
Thus, in addition to equipping the emergency department and intensive care unit with a TTE, we recommended that the portable TTE should also be routinely available in the ambulance, and according to the flowchart in Figure 3, emergency physicians need to make full use of the ADD risk score system and simultaneously detect the ascending aorta diameter to screen A-AAD early and rapidly in patients presenting with acute chest pain, especially in patients with AMI secondary to A-AAD.
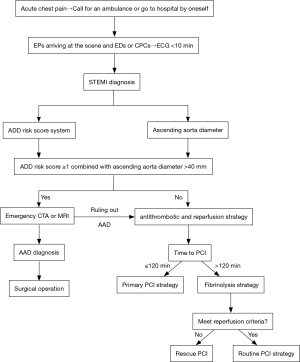
However, this study had several limitations. On the one hand, the results of our study might have some biases because this was a single-center retrospective study with the relatively small number of patients. To overcome this limitation, a series of prospective multicenter studies with large samples to evaluate this novel model (ADD risk score ≥1 combined with ascending aorta diameter >40 mm) should be urgently considered. On the other hand, potent evidence-based proof showing the necessity of equipping the portable TTE in the ambulance is still missing. Therefore, multicenter studies concerning the cost-benefit ratio of the portable TTE routinely available in the ambulance should also be urgently considered.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (No. 81470471).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Shanghai General Hospital (No. 2017KY233).
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation 2005;112:3802-13. [Crossref] [PubMed]
- Chen A, Ren X. Aortic Dissection Manifesting as ST-Segment-Elevation Myocardial Infarction. Circulation 2015;131:e503-4. [Crossref] [PubMed]
- Rogers AM, Hermann LK, Booher AM, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation 2011;123:2213-8. [Crossref] [PubMed]
- Nazerian P, Giachino F, Vanni S, et al. Diagnostic performance of the aortic dissection detection risk score in patients with suspected acute aortic dissection. Eur Heart J Acute Cardiovasc Care 2014;3:373-81. [Crossref] [PubMed]
- Sobczyk D, Nycz K. Feasibility and accuracy of bedside transthoracic echocardiography in diagnosis of acute proximal aortic dissection. Cardiovasc Ultrasound 2015;13:15. [Crossref] [PubMed]
- Pare JR, Liu R, Moore CL, et al. Emergency physician focused cardiac ultrasound improves diagnosis of ascending aortic dissection. Am J Emerg Med 2016;34:486-92. [Crossref] [PubMed]
- Cecconi M, Chirillo F, Costantini C, et al. The role of transthoracic echocardiography in the diagnosis and management of acute type A aortic syndrome. Am Heart J 2012;163:112-8. [Crossref] [PubMed]
- Nazerian P, Vanni S, Castelli M, et al. Diagnostic performance of emergency transthoracic focus cardiac ultrasound in suspected acute type A aortic dissection. Intern Emerg Med 2014;9:665-70. [Crossref] [PubMed]
- Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009;119:2702-7. [Crossref] [PubMed]
- Nazerian P, Morello F, Vanni S, et al. Combined use of aortic dissection detection risk score and D-dimer in the diagnostic workup of suspected acute aortic dissection. Int J Cardiol 2014;175:78-82. [Crossref] [PubMed]
- Gorla R, Erbel R, Kahlert P, et al. Accuracy of a diagnostic strategy combining aortic dissection detection risk score and D-dimer levels in patients with suspected acute aortic syndrome. Eur Heart J Acute Cardiovasc Care 2017;6:371-8. [Crossref] [PubMed]
- Neri E, Barabesi L, Buklas D, et al. Limited role of aortic size in the genesis of acute type A aortic dissection. Eur J Cardiothorac Surg 2005;28:857-63. [Crossref] [PubMed]
- Pape LA, Tsai TT, Isselbacher EM, et al. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007;116:1120-7. [Crossref] [PubMed]
- Meredith EL, Masani ND. Echocardiography in the emergency assessment of acute aortic syndromes. Eur J Echocardiogr 2009;10:i31-9. [Crossref] [PubMed]
- Cozijnsen L, Braam RL, Waalewijn RA, et al. What is new in dilatation of the ascending aorta? Review of current literature and practical advice for the cardiologist. Circulation 2011;123:924-8. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection. Eur Heart J 2001;22:1642-81. [Crossref] [PubMed]
- Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [PubMed]


