Current PD-L1 immunohistochemistry for non-small cell lung cancer
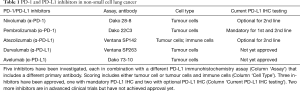
Immunotherapy that targets PD-1 or PD-L1 by monoclonal antibodies has emerged as effective and safe new treatment of malignant tumours. It is most beneficial in highly immunogenic tumours, in particular in malignant melanoma (1) and in non-small cell lung cancer (NSCLC) (2). Immunohistochemistry (IHC) of PD-L1 protein may serve as predictive biomarker for both PD-1 and PD-L1 inhibitors. In NSCLC, five different inhibitors have been developed in parallel, each with own PD-L1 IHC assay and interpretation criteria (3). Three inhibitors have achieved clinical approval which raises the need to investigate the comparability of the different PD-L1 assays (Table 1).
Full table
In a recent review in the Journal of Clinical Oncology, a group of renowned pathologists lead by Professors Reinhard Büttner and Ming-Sound Tsao provides a systematic overview of PD-L1 testing in NSCLC (4): the PD-L1 protein is expressed by both carcinoma cells and tumour-infiltrating immune cells (ICs). Since the predictive value differs all PD-L1 IHC assays have cell-type specific interpretation guidelines. In NSCLC, the scoring of carcinoma cells, also called ‘tumour cells’ (TC) and the scoring of IC are distinguished without further subclassification of the ICs (3).
PD-1 inhibitor Pembrolizumab is approved for first-line NSCLC-treatment both by the American (FDA) and European (EMA) drug administration agencies. PD-L1 IHC testing is required and patients with ≥50% stained TCs are eligible for Pembrolizumab monotherapy. Testing in the underlying clinical trials was done with the Dako 22C3 pharmDx assay.
In second-line NSCLC-treatment Pembrolizumab as well as PD-1 inhibitor Nivolumab and PD-L1 inhibitor Atezolizumab are approved. Pembrolizumab is indicated for patients with ≥1% stained TC. Nivolumab and Atezolizumab are available for all second-line patients and optional PD-L1 testing is possible using assays Dako 28-8 pharmDx and Ventana SP142. Dako 28-8 measures TCs and uses cut-offs ≥1%, ≥5% and ≥10%. SP142 includes TC scoring with a cut-off of ≥50% stained cells and IC scoring with a cut-off of ≥10% stained area (3).
PD-L1 inhibitors Durvalumab and Avelumab are under investigation for NSCLC but have not been approved yet.
Most evidence available indicates that the test performances of Dako 22C3 and Dako 28-8 are highly comparable. The same applies for Ventana SP263 assay which was originally developed for Durvalumab. Some studies show that SP263 might stain slightly higher proportions of TCs. However, SP263 has received ‘CE’-markings to make predictions for both Pembrolizumab and Nivolumab indicating that the difference might not be clinically relevant. On the other hand, Ventana SP142 shows a different type of PD-L1 staining that is not comparable to 22C3, 28-8 and SP263. Thus, testing for Atezolizumab has to be done specifically with SP142. The same may be true for Dako 73-10 assays; however, this assay is not yet commercially available. It may become of importance when the respective trials for Avelumab have been successfully completed.
Concerning practical application, the studies show that reliable PD-L1 testing is possible, i.e., satisfying interobserver and interlaboratory concordance can be achieved for tumour cell scoring. PD-L1 scoring does have its peculiarities and the interobserver concordance was found to be higher among pathologist who had received specific training. IC scoring was found to have lower concordance rates which could be improved by specific training. Still it seems to be more challenging to standardise compared to tumour cell scoring.
Finally, several studies have shown that it is possible to set up laboratory developed tests (LDTs) that match the staining results of the PD-L1 IHC assays. However, careful calibration and validation is essential. It seems to be easy to miss the appropriate measurement range and set up a LDT that does not show similar staining patterns and therefor is likely not to have the same predictive value. These results are reflected data of external quality assessment programs such as NordiQC which indicate that LDTs may be successful but have higher failure rates (5).
The review constitutes the most detailed overview of PD-L1 testing in NSCLC at the moment. With its focus on the practical aspects, it is a helpful resource in selecting the appropriate PD-L1 assay and in interpreting and reporting the result. Another publication that provides more background information on the different PD-L1 assays used in NSCLC is the ‘PD-L1 atlas’ by the International Association for the Study of Lung Cancer (IASLC) (3).
At present the role of PD-L1 in NSCLC and the modalities of its application are quite clear, but the potential of immunotherapy in NSCLC has just begun to unfold and required predictive biomarkers will develop further:
- A multitude of clinical trials for immunotherapy of NSCLC are ongoing which will likely yield more approvals of PD-1/PD-L1 inhibitors and of combinatory treatments. New approvals might include PD-L1 assays SP142 and Dako 73-10 as obligatory tests;
- Novel biomarkers might be included that supplement PD-L1 testing. Among these, expression analysis of the microenvironment (6) and the tumour mutational burden (7) are possible candidates.
Thus, it is an ongoing task for clinical pathology to follow, investigate and shape the biomarker program for NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: Scheel AH has collaborations with NordiQC (Aalborg, Denmark) and QuIP (Berlin, Germany) and has participated in advisory boards for Roche Pharmaceuticals, Bristol-Myers Squibb and Merck Sharp & Dohme within the past twelve months. Schäfer SC has no conflicts of interest to declare.
References
- Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017;390:1853-62. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Dacic S, et al., editors. IASLC Atlas of PD-L1 Immunohistochemistry testing in lung cancer, 1st edition 2017. International Association for the Study of Lung Cancer, Aurora, USA.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Büttner R, Gosney JR, Skov BG, et al. PD-L1 immunohistochemistry testing: a review on analytical assays and clinical implementation in non-small cell lung cancer. J Clin Oncol 2017;35:3867-76. [Crossref] [PubMed]
- NordiQC results for quality assessment of PD-L1 in NSCLC, run 'C1' 2017, retrieved 2018-01-10. Available online: http://www.nordiqc.org/downloads/assessments/96_102.pdf
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]


