In vitro assessment of cefoperazone-sulbactam based combination therapy for multidrug-resistant Acinetobacter baumannii isolates in China
Introduction
Acinetobacter baumannii (A. baumannii) is an important pathogen of nosocomial infection and can cause a wide range of infections including bacteremia, pneumonia, urinary tract infections, and wound infections (1,2). Antibiotic use and invasive procedures increase drug resistance and tolerance of A. baumannii (3). Particularly, the emergence of multi-drug resistant A. baumannii (MDRAB) presents a series of challenges to clinical anti-infection treatment (4,5), including high rate of failure and large costs. In intensive care unit (ICU) patients, the digestive tract is an important epidemiological reservoir for MDRAB infections in hospital outbreaks (6). MDRAB is disseminated worldwide (6,7) and is highly resistant to a number of available antibiotics, including aminoglycosides, quinolones, penicillin, cephalosporin, and carbapenems. At present, colistin and tigecycline have been employed as alternative therapeutic options for MDRAB infections. However, emergence of resistance to these antimicrobial agents has also been reported (8). Notwithstanding, combination therapy has been considered superior to single-drug therapy against MDRAB, with regards to both efficacy and lower risk of adverse reactions and drug toxicity (9-11). Tigecycline based therapy with various combinations such as cefoperazone-sulbactam, carbapenem, quinolone, or aminoglycoside antibiotics, has been adopted for treatment of MDRAB infections (12,13). However, the most effective combination therapy to treat A. baumannii infection has yet to be explored.
Cefoperazone is a bactericidal beta lactam antibiotic (14) that is commonly used in combination with a β-lactamase inhibitor, such as sulbactam, to enhance the activity of cefoperazone by irreversible inactivation of β-lactamases (15). In the absence of tigecycline, either cefoperazone-sulbactam or rifampicin is frequently prescribed to treat MDRAB infections, as both provide good antimicrobial effects against such infections (16,17). Tigecycline and rifampicin are good therapeutic options since they have no cross-resistance influence from β-lactam antibiotics. In addition, β-lactamase inhibitors, including sulbactam and tazobactam, are also effective in treating MDRAB infections (18). Moreover, rifampicin and cefoperazone-sulbactam in combination have synergistic effects against A. baumannii infections (19). The aim of the present study was to investigate the efficacy of cefoperazone-sulbactam combined with either tigecycline or rifampicin against clinical isolates of A. baumannii.
Methods
Collection and identification of bacteria
MDRAB (n=103) were clinically isolated from patients at Qilu Hospital at Shandong University and at The Second Affiliated Hospital of Shandong University of Chinese Medicine between December 2015 and July 2016. Of the 103 isolates, 38 were isolated from patients in the Department of Respiratory Medicine, 58 were collected from the ICU and 7 were obtained from the Neurosurgery Department. For the 103 isolates, 29% of them were obtained from bronchoalveolar lavage fluid, 2% from blood, and the rest from sputum. The Ethics Committee of Qilu Hospital at Shandong University approved this study [KYLL-2016 (KS)-507]. All participants in this study provided informed consent. All bacteria were identified using BBL Crystal Identification Kit (Becton Dickinson Diagnostics, Sparks, MD., USA) according to the manufacturer’s guidelines. Briefly, bacterial cultures were inoculated into the test kit, and then incubated for 4 h at 35 °C. Catalase, indol-spot, and gram stain tests were analyzed with the Crystal Mind software. MDRAB refers to isolates of A. baumannii that are non-susceptible to at least one agent in three or more antimicrobial categories, such as aminoglycosides, carbapenems, fluoroquinolones, penicillin and β-lactamase inhibitors, extended-spectrum cephalosporins, folate pathway inhibitors, polymyxins, and tetracyclines (20,21).
Antibiotic susceptibility testing
Minimum inhibitory concentration (MIC) values were assessed for tigecycline, ceftazidime, cefepime, cefoperazone, gentamycin, meropenem, levofloxacin, rifampicin, and amikacin for multi-drug resistant A. baumannii bacteria using the Epsilomer test (E test) method following clinical and laboratory standards institute (CLSI) guidelines. Briefly, 100 µL bacterial suspensions were spread on Muller-Hinton agar plates, E test strips (Sigma) were placed, and the plates were incubated for 24 h at 37 °C. MIC values were recorded according to CLSI guidelines, where MIC values ≤16/8 and ≥64/32 µg/mL of cefoperazone-sulbactam against A. baumannii are considered sensitive and resistant, respectively. The MIC for 90% of MDRAB (MIC90) was also recorded.
Synergy test
Synergy tests were performed using the E test method for each clinical isolate. The combination included: meropenem with rifampicin, amikacin, or cefoperazone-sulbactam; tigecycline with ceftazidime or ciprofloxacin; and cefoperazone-sulbactam with rifampicin. Briefly, strip A and strip B were placed crosswise, with the intersection of the MIC value of each antibiotic. Fractional inhibitory concentration index (FICI) was used to assess the effect of combination therapy. FICI was calculated as (MICa combination/MICa alone) + (MICb combination/MICb alone). MICa and MICb represent the MIC value read from strip A and strip B tests, respectively. A calculated FICI ≤0.5 represented a synergistic effect (22), a value between 0.5–1 represented as an additive effect, a value between 1–4 represented as an indifferent effect, and a value of >4 represented an antagonistic effect (22). Furthermore, the MIC value of each antibiotic was recorded during combination antimicrobial susceptibility tests.
Statistical analysis
The differences in synergistic and additive efficiency among different combination regimens were compared using the chi-square test. In the efficiency analysis of cefoperazone-sulbactam in combination with tigecycline or rifampicin, MDRAB were first grouped according to the extent of resistance to a single antibiotic, then the difference in synergistic and additive efficiency between different groups were tested using the chi-square test. A P value of <0.05 was considered significantly different.
Results
Bacterial identification and susceptibility pattern
All 103 bacteria were characterized as A. baumannii by the BBL Crystal Identification Kit and were classified as multidrug resistant. More than 95% of the isolates were resistant to ceftazidime, cefepime, gentamycin, meropenem, levofloxacin, and amikacin. Approximately 17.5% of the isolates were resistant to tigecycline, and the MIC50 and MIC90 were calculated as 2 and 8 µg/mL, respectively. Nearly 84.2% of the isolates were resistant to rifampicin, and the MIC50 and MIC90 were calculated as 4 and 16 µg/mL, respectively. However, approximately 90.8% of the isolates were resistant to cefoperazone-sulbactam, and the MIC50 and MIC90 were calculated as 32 and 128 µg/mL, respectively (Table 1).
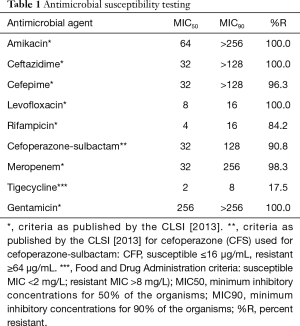
Full table
Evaluation of effective combination therapy against MDRAB
To identify the best drug combination with the highest efficacy, synergy tests were performed using the E test method. Synergistic effects were observed for the tigecycline and cefoperazone-sulbactam combination (66%), followed by rifampicin with cefoperazone-sulbactam (45.7%), and meropenem with cefoperazone-sulbactam (20.4%). No antagonistic effect was observed in any of the antibiotic combinations tested (Table 2).
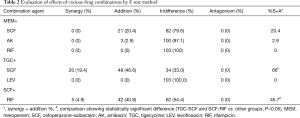
Full table
MIC value of tigecycline and cefoperazone-sulbactam in combination
Based on the MIC values of tigecycline, all 103 MDRAB were divided into four groups (≤1, 1–2, 2–4, and >4). The increased synergistic and additive effects of tigecycline and cefoperazone-sulbactam in combination were associated with higher tigecycline MIC values. A decreased MIC value of tigecycline was observed when used in combination. However, in strains with higher resistance, drug combination did not significantly decrease the MIC values below drug max concentration (Cmax) after combination. Similarly, based on the MIC value of cefoperazone-sulbactam, all bacterial strains were divided into three groups (32–64, 64–128, and >128). The MIC values of cefoperazone-sulbactam in all groups declined below Cmax when used in combination (Table 3). Therefore, the level of resistance to tigecycline was recognized as the limiting factor for effective tigecycline and cefoperazone-sulbactam combination therapy.
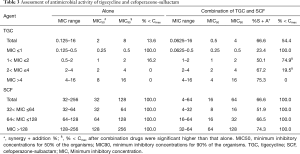
Full table
MIC value of rifampicin and cefoperazone-sulbactam in combination
Based on the MIC value of rifampicin, all 103 MDRAB were divided into four groups (≤4, 4–8, 8–16, and >16). Similar to the combination of tigecycline and cefoperazone-sulbactam, higher rifampicin MIC values resulted in better synergistic and additive effects of rifampicin and cefoperazone-sulbactam in combination. However, strains with higher MIC values for rifampicin had greater difficultly in decreasing the MIC values below Cmax after combination. Correspondingly, all bacterial strains were divided into three groups (32–64, 64–128, and >128) based on the different levels of resistance to cefoperazone-sulbactam. The MIC values of cefoperazone-sulbactam in all groups decreased below Cmax when used in combination (Table 4). Therefore, the level of resistance to rifampicin was the limiting factor for effective rifampicin and cefoperazone-sulbactam combination.
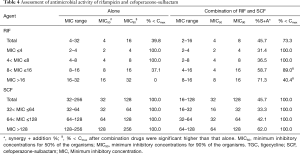
Full table
Discussion
MDRAB has emerged as a serious challenge for clinical anti-infection treatment due to acquired resistance to most of the previously existing antibiotics (4,5). This emerging resistance could be explained by the increased application of single-drug antibiotics. However, the most effective combination therapy to treat A. baumannii infection is still unclear. In the present study, tigecycline and cefoperazone-sulbactam combination had the greatest synergistic effect in most MDRAB isolates in vitro. The increased synergistic and additive effects of tigecycline and cefoperazone-sulbactam in combination were enhanced by higher tigecycline MIC values.
Tigecycline is a new class of antibiotic that has an ammonia acyl ring element and exerts a strong antibacterial effect against carbapenem-resistant MDRAB (23,24). Because the Cmax was only 0.72±0.24 µg/mL at the common, normal dose (100 mg initial dose, followed by 50 mg per every 12 h) (25), there is an increased chance that drug resistance will develop with long-term application of tigecycline. Therefore, tigecycline should be used in combination with other antibiotics for treating serious MDRAB infections. In the current study, the combination of tigecycline and cefoperazone-sulbactam showed the best synergetic antimicrobial effect against MDRAB, which is in accordance with reports by Liu et al. (4,15), who reported a 29% synergistic effect for the combination therapy. Moreover, tigecycline in combination with cefoperazone-sulbactam showed a more significant effect than tigecycline in combination with sulbactam against MDRAB. It is worth noting that the bacterial drug resistance level significantly impacted the combination effect. Although the synergistic and additive effects of tigecycline and cefoperazone-sulbactam in combination increased with higher tigecycline MIC values, the MIC value was still higher than the Cmax in the combination therapy. Therefore, the common doses of tigecycline, either administered singularly or in combination, are not sufficient to treat highly resistant bacterial strains (MIC >4).
Rifampicin can be used to effectively treat pneumonia in a mouse model infected with drug resistant A. baumannii (26); however, the singular use of rifampicin often results in drug resistance. It is reported that rifampicin alone leads to drug resistance after 24 h treatment of MDRAB infection (27). Thus, rifampicin should be used in combination with other antibiotics. Previous studies showed that combination of rifampicin with colistin or carbapenem produced a synergistic effect when used to treat drug resistant Pseudomonas aeruginosa, Klebsiella bacillus and A. baumannii infections (18,28). Rifampicin in combination was also reported to significantly decrease MIC values (29). In our study, the combination of rifampicin with cefoperazone-sulbactam decreased the synergistic effect more than the combination of tigecycline with cefoperazone-sulbactam. However, these combinations may also be used as alterative option for MDRAB infection, especially for those bacteria with a lower degree of resistance (30,31). The degree of antimicrobial resistance can affect the result of combination effects. The MIC values of tigecycline or rifampicin in combination are still higher than Cmax, which may explain why the combination is less effective against high drug resistant strains clinically. In addition, the single-drug (tigecycline or rifampicin) MIC value can also be used for predicting prognosis after drug combination. It is worth mentioning that one shortcoming of the study is that it lacks in vivo animal experiments. Further research is in progress to evaluate the effect of the cefoperazone-sulbactam based combinations in MDRAB infection animal experiments.
In conclusion, in vitro cefoperazone-sulbactam in combination with tigecycline or rifampicin produced the highest synergistic or additive effects against multi-drug resistant A. baumannii. However, due to the low Cmax of tigecycline and rifampicin, these combinations might work better for bacteria with moderate or low drug resistance levels.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee of Qilu Hospital at Shandong University approved this study [KYLL-2016 (KS)-507]. All participants in this study provided informed consent.
References
- El-Ageery SM, Abo-Shadi MA, Alghaithy AA, et al. Epidemiological investigation of nosocomial infection with multidrug-resistant Acinetobacter baumannii. Eur Rev Med Pharmacol Sci 2012;16:1834-9. [PubMed]
- Bachoumas K, Lebert C, Lacherade JC, et al. Community-acquired Acinetobacter baumannii pneumonia. Med Mal Infect 2015;45:337-9. [Crossref] [PubMed]
- Lin MF, Lan CY. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J Clin Cases 2014;2:787-814. [Crossref] [PubMed]
- Dong X, Chen F, Zhang Y, et al. In vitro activities of sitafloxacin tested alone and in combination with rifampin, colistin, sulbactam, and tigecycline against extensively drug-resistant Acinetobacter baumannii. Int J Clin Exp Med 2015;8:8135-40. [PubMed]
- Fan B, Guan J, Wang X, et al. Activity of Colistin in Combination with Meropenem, Tigecycline, Fosfomycin, Fusidic Acid, Rifampin or Sulbactam against Extensively Drug-Resistant Acinetobacter baumannii in a Murine Thigh-Infection Model. PLoS One 2016;11:e0157757. [Crossref] [PubMed]
- Jiang M, Liu L, Ma Y, et al. Molecular Epidemiology of Multi-Drug Resistant Acinetobacter baumannii Isolated in Shandong, China. Front Microbiol 2016;7:1687. [Crossref] [PubMed]
- Gulen TA, Guner R, Celikbilek N, et al. Clinical importance and cost of bacteremia caused by nosocomial multi drug resistant acinetobacter baumannii. Int J Infect Dis 2015;38:32-5. [Crossref] [PubMed]
- Quan J, Li X, Chen Y, et al. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect Dis 2017;17:400-10. [Crossref] [PubMed]
- Tangden T. Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Ups J Med Sci 2014;119:149-53. [Crossref] [PubMed]
- Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 2012;25:450-70. [Crossref] [PubMed]
- Petite SE, Bauer SR, Bollinger JE, et al. Antimicrobial Monotherapy versus Combination Therapy for the Treatment of Complicated Intra-Abdominal Infections. Pharmacotherapy 2016;36:1138-44. [Crossref] [PubMed]
- Trabelsi B, Trifa M, Ben Khalifa S. Tigecycline-based therapy for glycopeptide-resistant Enterococcus faecium infection in a pediatric intensive care unit. Tunis Med 2016;94:336-7. [PubMed]
- Chuang YC, Cheng CY, Sheng WH, et al. Effectiveness of tigecycline-based versus colistin- based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumannii in a critical setting: a matched cohort analysis. BMC Infect Dis 2014;14:102. [Crossref] [PubMed]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008;21:538-82. [Crossref] [PubMed]
- Liu B, Bai Y, Liu Y, et al. In vitro activity of tigecycline in combination with cefoperazone-sulbactam against multidrug-resistant Acinetobacter baumannii. J Chemother 2015;27:271-6. [Crossref] [PubMed]
- Song JY, Cheong HJ, Lee J, et al. Efficacy of monotherapy and combined antibiotic therapy for carbapenem-resistant Acinetobacter baumannii pneumonia in an immunosuppressed mouse model. Int J Antimicrob Agents 2009;33:33-9. [Crossref] [PubMed]
- Montero A, Ariza J, Corbella X, et al. Efficacy of colistin versus beta-lactams, aminoglycosides, and rifampin as monotherapy in a mouse model of pneumonia caused by multiresistant Acinetobacter baumannii. Antimicrob Agents Chemother 2002;46:1946-52. [Crossref] [PubMed]
- Carmeli Y, Akova M, Cornaglia G, et al. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clin Microbiol Infect 2010;16:102-11. [Crossref] [PubMed]
- Cetin ES, Tekeli A, Ozseven AG, et al. Determination of in vitro activities of polymyxin B and rifampin in combination with ampicillin/sulbactam or cefoperazone/sulbactam against multidrug-resistant Acinetobacter baumannii by the E-test and checkerboard methods. Jpn J Infect Dis 2013;66:463-8. [Crossref] [PubMed]
- APIC. Guide to the elimination of multidrug -resistant acinetobacter baumannii transmission in healthcare settings. Available online: http://wwwapicorg/EliminationGuides, 2010.
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268-81. [Crossref] [PubMed]
- Mohammadi M, Khayat H, Sayehmiri K, et al. Synergistic Effect of Colistin and Rifampin Against Multidrug Resistant Acinetobacter baumannii: A Systematic Review and Meta-Analysis. Open Microbiol J 2017;11:63-71. [Crossref] [PubMed]
- Metan G, Alp E, Yildiz O, et al. Clinical experience with tigecycline in the treatment of carbapenem-resistant Acinetobacter infections. J Chemother 2010;22:110-4. [Crossref] [PubMed]
- Shankar C, Nabarro LEB, Anandan S, et al. Minocycline and Tigecycline: What Is Their Role in the Treatment of Carbapenem-Resistant Gram-Negative Organisms? Microb Drug Resist 2017;23:437-46. [Crossref] [PubMed]
- Conte JE Jr, Golden JA, Kelly MG, et al. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int J Antimicrob Agents 2005;25:523-9. [Crossref] [PubMed]
- Pachon-Ibanez ME, Fernandez-Cuenca F, Docobo-Perez F, et al. Prevention of rifampicin resistance in Acinetobacter baumannii in an experimental pneumonia murine model, using rifampicin associated with imipenem or sulbactam. J Antimicrob Chemother 2006;58:689-92. [Crossref] [PubMed]
- Belet N, Haciomeroglu P, Kucukoduk S. Ciprofloxacin treatment in newborns with multi-drug-resistant nosocomial Pseudomonas infections. Biol Neonate 2004;85:263-8. [Crossref] [PubMed]
- Tripodi MF, Durante-Mangoni E, Fortunato R, et al. Comparative activities of colistin, rifampicin, imipenem and sulbactam/ampicillin alone or in combination against epidemic multidrug-resistant Acinetobacter baumannii isolates producing OXA-58 carbapenemases. Int J Antimicrob Agents 2007;30:537-40. [Crossref] [PubMed]
- Wareham DW, Bean DC. In-vitro activity of polymyxin B in combination with imipenem, rifampicin and azithromycin versus multidrug resistant strains of Acinetobacter baumannii producing OXA-23 carbapenemases. Ann Clin Microbiol Antimicrob 2006;5:10. [Crossref] [PubMed]
- Pongpech P, Amornnopparattanakul S, Panapakdee S, et al. Antibacterial activity of carbapenem-based combinations againts multidrug-resistant Acinetobacter baumannii. J Med Assoc Thai 2010;93:161-71. [PubMed]
- Yoon J, Urban C, Terzian C, et al. In vitro double and triple synergistic activities of Polymyxin B, imipenem, and rifampin against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2004;48:753-7. [Crossref] [PubMed]


