Non-intubated thoracoscopic bullectomy under sedation is safe and comfortable in the perioperative period
Introduction
Thoracoscopic bullectomy is a simple, relatively short duration thoracic surgical procedure. However, it requires use of a double lumen endotracheal tube or single lumen tube with bronchial blocker. It takes a longer time to confirm correcting positioning of a double lumen endotracheal tube or single lumen tube with bronchial blocker when performing one-lung ventilation than two-lung ventilation. Furthermore, due to the double lumen endotracheal tube’s large diameter and difficulty in manipulating it in the airway, patients complain of sore throat, pain, and hoarseness despite the short duration of the operation (1,2). Tracheal laceration has also been reported after insertion of a double lumen tracheal tube (3). Therefore, we hypothesized that non-intubated thoracoscopic bullectomy under sedation would be preferable to that under general anesthesia.
Many researchers and thoracic surgeons have examined and reported on cases of non-intubated thoracoscopic bullectomy. However, in most of these studies, operations were performed under epidural anesthesia with or without sedation (4-7), even though insertion of an epidural catheter can increase comorbidities such as dural puncture headache, epidural bleeding, infection, and spinal cord injury. Thus, in this study, we evaluated the efficacy of non-intubated thoracoscopic bullectomy under sedation with dexmedetomidine and ketamine and compared it with that of general anesthesia using a double lumen endotracheal tube.
Methods
This study was designed in a prospective, randomized, double-blinded, parallel manner and approved by the Committee on Clinical Investigation for human research (IRB) at Korea University Ansan Hospital (123, Jeokgeum-ro, Danwon-gu, Ansan-si, Gyeonggi-do, 425-707, South Korea, AS12130-002, 2012.11.20).
All subjects and/or legal guardians gave signed informed consent to participate. During the study, subjects and nurses who investigated postoperative pain and discomfort were blinded to anesthetic methods. The study is registered at ClinicalTrials.gov (NCT02109510).
The study was performed between September 2014 and May 2015, with 41 patients who were to undergo thoracoscopic bullectomy for primary spontaneous pneumothorax. All primary spontaneous pneumothorax patients scheduled for video assisted thoracoscopic bullectomy were eligible to participate. Primary spontaneous pneumothorax is defined as a pneumothorax without any clinical lung disease such as chronic obstructive lung disease, interstitial lung disease, pulmonary tuberculosis, or other.
Patients with medical conditions that contraindicate the use of general anesthesia and patients who were not able to express postoperative pain and discomfort subjectively were excluded from the study (Table 1). History of pneumothorax was not an exclusion criterion. However, patients with a history of ipsilateral lung surgery were excluded because pleural adhesion was anticipated in these cases.
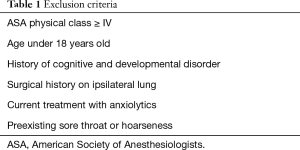
Full table
Anesthesia
A random number table and the sealed envelope technique were used to determine the assignments to one of the two groups: the sedation anesthesia group (SA group) (sedation without tracheal intubation) or the general anesthesia group (GA group) (general anesthesia with tracheal intubation using a 35- or 37- Fr left-sided double lumen tracheal tube). All patients were fasted for more than 8 hours before surgery and were premedicated with midazolam 2 mg and glycopyrrolate 0.2 mg intramuscular injection. After entering the operating room, monitors were placed on patients including an EKG, a pulse oximeter, an automated noninvasive pressure device, or bispectral index (BIS) to monitor the depth of anesthesia. In SA group, dexmedetomidine was diluted in 2 µg/mL in normal saline and was administered at a loading dose of 1 µg/kg for 10 min and then maintained in dosages of 0.3–1 µg/kg/h. Also, ketamine was infused at a rate of 2–4 mg/kg/h intraoperatively. Oxygen (O2) supply was maintained through a non-rebreather mask (oxygen flow 12 L/min, FiO2 =0.5). Sedation level was maintained between Ramsay sedation score 5 and 6.
For GA patients, propofol 2 mg/kg and rocuronium 0.6 mg/kg were IV injected to induce anesthesia and then double lumen endotracheal intubation was performed. Anesthesia was maintained with 1.0–2.5% sevoflurane and mixture of O2 and medical air at a fresh gas flow of 2 L/min.
In both groups, perioperative arterial blood gas analysis was taken before one-lung ventilation, during one-lung ventilation, and after two-lung ventilation.
Surgical procedure
In the two groups, thoracoscopic bullectomy was performed in the same manner and all operations were conducted by single surgeon. After induction, the patient was positioned in a lateral decubitus position. Prepping and draping were done in a sterile manner. Thoracoscopic bullectomy was performed through a 1.7-cm single port. At the beginning of the surgery, 20 mL of 2% lidocaine was instilled around the port site for postoperative analgesia and intrathoracic vagal block was performed under thoracoscopic view. Bullae on visceral pleura were resected using endoscopic staplers. For surgical drainage, a 24 Fr chest tube was inserted through the port. At the end of the operation, pressure-controlled suction devices were applied to expand the collapsed lung.
Data collection and statistics
We recorded the time for anesthesia [including emergence time and post-anesthesia care unit (PACU) recovery time] and operation, postoperative pain, sore throat, hoarseness, adverse events (nausea, vomiting, hypotension and bradycardia), dose of rescue analgesic drug used for 24 hours post-operatively and perioperative arterial blood gas analysis to evaluate the efficacy.
At 1 and 24 hours after operation, the patients were asked to rate their pain a 0–10 centimeters visual analog scale (VAS), where a score of 0 meant no pain and a score of 10 represented the worst pain imaginable. The use of a VAS to measure discomfort has been validated for chronic pain (8,9) and acute postoperative pain (10). The occurrences of sore throat and hoarseness were recorded and their intensities were assessed using the following scores: 0, none; 1, mild; 2, moderate; 3, severe. Hoarseness was defined as a change in voice quality. Diclofenac 30 mg was injected intramuscularly when a patient complained of severe post-operative pain (VAS >8/10), and the usage of diclofenac for 24 hours post-operatively was recorded.
The sample size was estimated from preliminary data obtained from 40 patients and our assumption was that a more than 25% or 2.5-point reduction in VAS score for sore throat would be clinically relevant. The power analysis suggested that a minimum of 20 patients in each group would be needed for a β=0.2 and α=0.05. Therefore, we enrolled 20 and 21 patients the GA and SA groups, respectively.
SPSS v.19.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. One-way ANOVAs were done to analyze differences between the case and control group for VAS score, sore throat, and hoarseness at 1 and 24 hours after surgery. And Fisher’s exact test was carried out to compare the incidences of adverse events between the two groups. We also conducted repeated measures ANOVA to compare PCO2, pH, and SpO2 according to the measurement time and the group and performed t-tests to evaluate the significance of group differences. P<0.05, <0.01, and <0.001 were taken to indicate statistical significance.
Results
Demographic data of patients
The characteristics of the population are shown in Table 2. Forty-one patients aged 19 to 52 years scheduled for thoracoscopic bullectomy were enrolled after providing written consent (GA: n=20, 48.8%; SA: n=21, 51.2%). Thirty-eight patients were male (92.7%) while the remaining 3 patients were female (7.3%). The mean age of the patients was 22.49 years. The number of patients requiring surgery on the right side was 16 (39.0%), while 24 (58.5%) required surgery on the left side and 1 (2.4%) needed surgery on both sides.
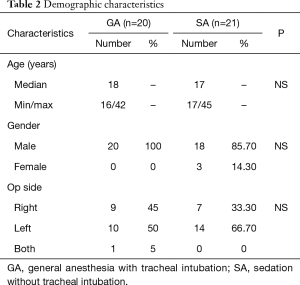
Full table
Surgical and anesthetic results
We compared the times required for anesthesia, surgery, emergence, and PACU recovery between the two groups using t-tests (Table 3). Surgery times did not differ significantly between the GA and SA groups (P=0.062). Collapse of the operative lung and view field of operation were satisfactory in both groups. Anesthetic time and emergence time were shorter in the SA group than in the GA group (P<0.05). In contrast, the PACU recovery time was longer in the SA group than in the GA group (P<0.05).
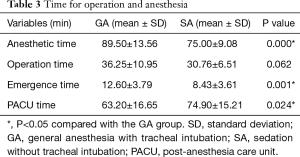
Full table
We compared VAS, sore throat and hoarseness scores at 1 and 24 hours postoperatively between the two groups (Table 4). VAS and sore throat scores at 1 hour were significantly lower in the SA group than in the GA group (VAS P=0.000, sore throat P=0.002). However, at 24 hours, there were no significant differences between the two groups (VAS P=0.069, sore throat P=0.056). Hoarseness did not differ between the two groups at 1 or 24 hours.
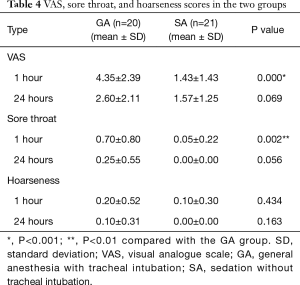
Full table
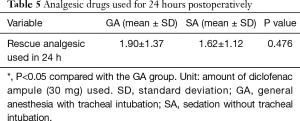
We measured the amounts of analgesic drugs used for 24 hours after surgery for each group (Table 5). The differences between the two groups in the analgesic requirements of the patients within 24 hours of surgery were not significant (P=0.476).
Full table
We compared perioperative PaCO2 values according to the measurement time point for each group (Figure 1A). During the pre- and intraoperative periods there were significant differences between the two groups (P<0.001)—namely, greater PaCO2 values were seen in the SA group than in the GA group. In the SA group, the intraoperative PaCO2 value was significantly elevated compared with the preoperative value (P<0.001), while the postoperative PaCO2 value did not differ significantly from the preoperative value (P>0.05). PaCO2 values in the postoperative period did not differ significantly between the two groups (P=0.131).

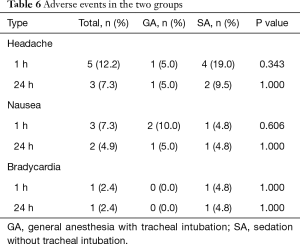
We compared perioperative PaO2 and SpO2 values according to the measurement time for each group (Figure 1B,C). Differences in PaO2 and SpO2 values between the two groups were only apparent in the intra-operative period (PaO2, P=0.012; SpO2, P=0.005). In the SA group, the intraoperative PaO2 value was significantly reduced compared with the preoperative value (P=0.004), but not with the postoperative PaO2 value (P=0.906). No significant differences were seen in the SA group for SpO2 throughout the surgery (intraoperative P=0.060, postoperative P=1.000). The value of pH was also maintained in normal range throughout the surgery and we could not any differences between two groups. The incidences of adverse events did not differ between the two groups (Table 6). Vomiting and hypotension were not presented in both groups.
Full table
Discussion
Non-intubated thoracic surgery (NITS) is a thoracic surgery that can be done without endotracheal intubation with regional block and sedation, or sedation only. Generally, thoracic surgery requires one-lung ventilation using a double lumen endotracheal tube or endobronchial blocker. However due to the tube’s large diameter and difficulty in manipulating it in the airway, patients often complain of sore throat and hoarseness after surgery (1,2). Knoll et al. (1) compared the incidence rates of airway injury from double lumen endotracheal tubes and bronchial blockers and reported that 44% of patients experienced hoarseness when double lumen endotracheal tubes were used. Moreover, many airway traumas are known to be associated with endotracheal intubation such as upper airway bleeding, tracheal laceration, and epiglottitis (3,11,12). In this study, sore throat was significantly lower in the SA group than the GA group, though there was no clear distinction in hoarseness between the two groups.
Recently, many researchers have reported that NITS has good feasibility and provides less side effects of general anesthesia, surgery-related trauma, and it also promotes recovery by maintaining a normal physiologic status (7,13-15). According to a survey in Europe, NITS was conducted more than 2,000 cases under various anesthetic methods (16). Recently Hung et al. (5) reported that NITS was safely done with a thoracic epidural block with or without sedation and an intercostal block. Several other studies of NITS confirmed the feasibility of surgeries under thoracic epidural block with propofol and remifentanil infusion (6,7,13,14,17). The thoracic epidural block might have some advantages to sedation, but also has disadvantages such as local anesthetic neurotoxicity, hypotension, bradycardia induced by sympatheticolysis, accidental dural puncture headache, infection, and neurological complications like spinal cord injury. Moreover, thoracic epidural anesthesia is also a technically demanding and time-consuming procedure (18,19). In our study, we thought that sedation using dexmedetomidine and ketamine would provide adequate anesthesia for NITS because thoracoscopic bullectomy is a simple, short procedure.
Considering that NITS has been safely conducted in high-risk patients such as extremely old people and those with multiple co-morbidities and severe respiratory dysfunction, we were confident that non-intubated thoracoscopic bullectomy would be safe to perform. During NITS, spontaneous one lung ventilation was achieved by surgical pneumothorax on non-dependent side of lung and the non-dependent side of lung did not inflate to opened cavity. The collapse of the operated side of lung was satisfactory for all kinds of procedures and it was not required capnothorax to secure the operation field. There were no conversions to GA in the SA group, and no significant adverse events in our study. In previous studies, the rates of conversion to GA have ranged from 2.6% to 10% with assorted causes (4-7,13,14). Anesthetic problems, like persistent hypoxemia and poor epidural anesthesia, as well as surgical problems like bleeding, dense adhesions between the lungs and diaphragm, and significant mediastinal movement have also been reported in other studies (4-7,13,14). These studies were performed primarily on lung cancer patients who had undergone more complicated surgeries like lobectomy, segmentectomy, and wedge resection rather than bullectomy. These results suggest that the status of the subject and the complexity of the procedure are important considerations when performing NITS.
Permissive hypercapnia and hypoxemia are commonly seen in NITS, and are consequences of a reduction in tidal volume by iatrogenic pneumothorax (20). In our study, the PaCO2 level in the SA group was elevated (mean 56.47 mmHg) at the time of one-lung ventilation (intra-op period), but considering that many studies describe a transient perioperative hypercapnia (<55 mmHg), this value is still in the tolerable range (21-24). Furthermore, after one-lung ventilation (postoperative period), CO2 retention in the SA group improved. And the PaO2 level in the SA group was also lower during the one-lung ventilation period (intraoperative period, mean 151.50 mmHg) than it was before one-lung ventilation (preoperative period, mean 219.08 mmHg), but the SPO2 level in the SA group did not change throughout the procedure. Only during the one-lung ventilation period showed differences in PaO2 and SPO2 between the SA group and the GA group, but the mean values of PaO2 (151.5 mmHg) and SPO2 (97.15%) were within the tolerable range and hypoxemia was not found in any patient. Therefore, in our study, hypercapnia and hypoxemia were not generally a barrier to performing NITS.
Ketamine is a non-competitive N-methyl-d-aspartate receptor antagonist with characteristics of sedation, amnesia, and less respiratory depression, and dexmedetomidine is a highly specific α2-adrenoceptor agonist with sedative, analgesic, and anxiolytic properties and does not show significant respiratory depression when used at a clinical dosage (25). In contrast, other sedatives like propofol and benzodiazepines can cause severe respiratory depression at clinical dosage. Therefore, we used dexmedetomidine and ketamine for sedation because we thought that the continuous infusion of dexmedetomidine and ketamine could preserve self-respiration better than the continuous infusion of propofol or benzodiazepines during operation (26,27). Moreover, in a comparison of dexmedetomidine and propofol for intraoperative sedation, dexmedetomidine showed a slower onset of sedation, but resulted in less pain during the postoperative period due to its analgesic-sparing effect (28). Combination use of dexmedetomidine and ketamine offsets the sympathoinhibitory effect of the former with the cardiostimulatory effects of the latter. Consequently, hemodynamic instabilities like hypotension, bradycardia, hypertension, and tachycardia happen less often when a combination of the drugs is used than when the drugs are used separately (29). In addition, the slow onset of sedation by dexmedetomidine could be shortened by conjunctive use of ketamine (30). Dexmedetomidine also blocks undesirable central nervous system effects like the post-anesthetic delirium effect of ketamine (31). In our study, we checked for hypotension and bradycardia, which are commonly seen when dexmedetomidine is used for sedation. Only one case of bradycardia was seen in the SA group.
VAS score, sore throat and hoarseness were the primary endpoints of our study. Pavlin et al. (32) reported that postoperative pain was the most common cause of early recovery delay (phase I), and that pain scores in the early recovery period were significantly correlated with patient comfort and recovery process. Delayed recovery time and prolonged anesthetic time can also have a significant impact on resource utilization for the medical team. In this study, the VAS score, and sore throat were less severe in the SA group than in the GA group in the early recovery period and anesthetic time is shorter in the SA group. It is possible that the analgesic effects of dexmedetomidine and ketamine might have influenced the postoperative VAS score (33-35). However, the context-sensitive half-time of dexmedetomidine is known to be 25 minutes after a 1-hour infusion (36) and the context-sensitive half-time of ketamine was similar to that of propofol. Moreover, the infusion of dexmedetomidine was ceased 15 minutes before the end of surgeries and we evaluated the VAS score of patients at the end of PACU stay. Therefore, we are confident that the recovery of patients who had bullectomy was promoted by NITS, not by the residual effect of dexmedetomidine and ketamine.
Limitation
Far more male patients than female patients were enrolled in this study. This could have affected the observed incidence of postoperative nausea/vomiting. Moreover, it is known that males are more susceptible to pneumothorax than females (37), which may explain why most patients in this study were male.
Conclusions
In conclusion, based on our results, non-intubation with sedation can be safely performed for bullectomy for primary spontaneous pneumothorax, and NITS can be performed with sedation only using dexmedetomidine and ketamine to prevent tracheal-induced comorbidities such as sore throat, hoarseness, and tracheal damage. Further, from the viewpoint of the patient, pain relief after NITS is more satisfactory.
Acknowledgements
Funding: This study was conducted by 2017 Korea University Ansan Hospital R&D support project through the support of Vice President for Medical Affairs of Korea University special research funds (No. O1700661)
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical approval for this study (AS12130-002, 2012.11.20) was provided by Korea University Medical Center Ansan Hospital Institutional Review Board (516, Gojan-dong, Danwon-gu, Ansan-si, Gyeonggi-do, 425-707, Korea). All subjects provided written informed consent prior to enrollment.
References
- Knoll H, Ziegeler S, Schreiber JU, et al. Airway injuries after one-lung ventilation: a comparison between double-lumen tube and endobronchial blocker: a randomized, prospective, controlled trial. Anesthesiology 2006;105:471-7. [Crossref] [PubMed]
- Zhong T, Wang W, Chen J, et al. Sore throat or hoarse voice with bronchial blockers or double-lumen tubes for lung isolation: a randomised, prospective trial. Anaesth Intensive Care 2009;37:441-6. [PubMed]
- Medina CR, Camargo Jde J, Felicetti JC, et al. Post-intubation tracheal injury: report of three cases and literature review. J Bras Pneumol 2009;35:809-13. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Gaston-Johansson F, Gustafsson M. Rheumatoid arthritis: determination of pain characteristics and comparison of RAI and VAS in its measurement. Pain 1990;41:35-40. [Crossref] [PubMed]
- Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 1993;55:195-203. [Crossref] [PubMed]
- DeLoach LJ, Higgins MS, Caplan AB, et al. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg 1998;86:102-6. [PubMed]
- Domino KB, Posner KL, Caplan RA, et al. Airway injury during anesthesia: a closed claims analysis. Anesthesiology 1999;91:1703-11. [Crossref] [PubMed]
- Benjamin B. Laryngeal trauma from intubation: Endoscopic evaluation and classification. In: Cummings CW, Fredrickson JM, Harker LA. editors. Otolaryngology Head & Neck Surgery, 3rd ed. St. Louis: Mosby, 1998:2013-35.
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [Crossref] [PubMed]
- Pompeo E, Sorge R, Akopov A, et al. Ests Non-intubated Thoracic Surgery Working Group. Non-intubated thoracic surgery-A survey from the European Society of Thoracic Surgeons. Ann Transl Med 2015;3:37. [PubMed]
- Guo Z, Shao W, Yin W, et al. Analysis of feasibility and safety of complete video-assisted thoracoscopic resection of anatomic pulmonary segments under non-intubated anesthesia. J Thorac Dis 2014;6:37-44. [PubMed]
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg 2007;32:13-9. [Crossref] [PubMed]
- Freise H, Van Aken HK. Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth 2011;107:859-68. [Crossref] [PubMed]
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery. Ann Transl Med 2014;2:106. [PubMed]
- Wagih M. Permissive hypercapnia: From the ICU to the operating room. Egypt J Cardiothorac Anesth 2014;8:1-4. [Crossref]
- Mineo TC, Pompeo E, Mineo D, et al. Awake nonresectional lung volume reduction surgery. Ann Surg 2006;243:131-6. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Frasca L, et al. Awake thoracoscopic bullaplasty. Eur J Cardiothorac Surg 2011;39:1012-7. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Fabbi E, et al. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [Crossref] [PubMed]
- Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology 2000;93:1345-9. [Crossref] [PubMed]
- Forster A, Gardaz JP, Suter PM, et al. Respiratory depression by midazolam and diazepam. Anesthesiology 1980;53:494-7. [Crossref] [PubMed]
- Koroglu A, Teksan H, Sagir O, et al. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg 2006;103:63-7. table of contents. [Crossref] [PubMed]
- Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461-6. [PubMed]
- Tammam TF. Comparison of the efficacy of dexmedetomidine, ketamine, and a mixture of both for pediatric MRI sedation. Egypt J Anaesth 2013;29:241-6. [Crossref]
- Tobias JD. Dexmedetomidine and ketamine: an effective alternative for procedural sedation? Pediatr Crit Care Med 2012;13:423-7. [Crossref] [PubMed]
- Levänen J, Makela ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology 1995;82:1117-25. [Crossref] [PubMed]
- Pavlin DJ, Chen C, Penaloza DA, et al. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg 2002;95:627-34. table of contents. [PubMed]
- Schnabel A, Meyer-Frießem CH, Reichl SU, et al. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain 2013;154:1140-9. [Crossref] [PubMed]
- Schnabel A, Reichl SU, Poepping DM, et al. Efficacy and safety of intraoperative dexmedetomidine for acute postoperative pain in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth 2013;23:170-9. [Crossref] [PubMed]
- Bell RF, Dahl JB, Moore RA, et al. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review). Acta Anaesthesiol Scand 2005;49:1405-28. [Crossref] [PubMed]
- Venn RM, Karol MD, Grounds RM. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive caret. Br J Anaesth 2002;88:669-75. [Crossref] [PubMed]
- Dotson K, Timm N, Gittelman M. Is spontaneous pneumothorax really a pediatric problem? A national perspective. Pediatr Emerg Care 2012;28:340-4. [Crossref] [PubMed]


