Effects of aging and comorbidities on nutritional status and muscle dysfunction in patients with COPD
Introduction
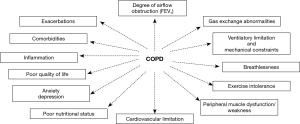
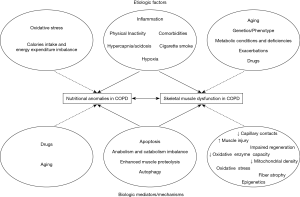
Chronic obstructive pulmonary disease (COPD) is a prevalent, complex and debilitating disease which imposes a formidable burden on patients and the healthcare system (1). The recognition that COPD is a multifaceted disease is not new, and increasing evidence have outlined the importance of its extra-pulmonary manifestations and its relation to other comorbid conditions in the clinical course of the disease and its societal cost (2). In particular, the association between COPD and skeletal muscle dysfunction/nutritional status anomalies has long been recognized, and the deleterious synergistic effects of their co-occurrence on clinical prognosis have been well established (3-10) (Figure 1). However, the fact that COPD is frequently associated with other conditions that also alter muscle function and nutritional status such as older age and chronic comorbid diseases intensify the need for a better understanding of the individual pathophysiological processes at play and their inter-relationships, in order to provide better clinical care (Figure 1). It is now evident that aging and comorbidities such as chronic heart failure (CHF) and chronic kidney disease (CKD), along with cigarette smoke, systemic inflammation, exercise, exacerbations, anabolic insufficiency and drugs play a relevant role in contributing to nutritional status and muscle dysfunction in patients with COPD (Figure 2). All the above factors modify the nutritional status and the phenotype of the muscles, through the induction of several biological phenomena in patients with COPD (Figure 2).
It has been 7 years since a research seminar have been totally devoted to this topic (11); since then, numerous researches have been undertaken to try to elucidate the effects of aging and comorbidities on nutritional status and muscle dysfunction in patients with COPD.
This review proposes to examine the current knowledge pertaining to the alterations in skeletal muscle function and nutritional status induced by COPD itself, and to compare them to those observed in physiological aging and in frequent chronic diseases associated with COPD, in order to delineate the independent and possibly additive contributions of each condition to the overall status of these patients.
Aging and skeletal muscular function/nutritional status in patients with COPD
In this section, we will focus on the effects of the aging process on skeletal muscle function and morphology and on the nutritional status, with a particular emphasis on the recent data supporting the consideration of COPD as a disease of accelerated aging.
Physiological consequences of the aging process on skeletal muscles
Sarcopenia (loss of muscle mass associated with a decline in function) is common in the elderly (12) and is characteristic of the physiological aging process (13,14). Changes in muscle morphology are therefore frequently observed with increasing age and relate to changes in muscle fibre composition, size and number (15,16). Numerous studies have investigated the changes in muscle fibre distribution in the skeletal muscles of elderly subjects, with sometimes contradicting results: an increase in the proportion of type 1 (slow-twitch) muscle fibres has frequently been described (17-21), although others report a relative stability (22-29), or even a decrease (30) in the proportion of type I fibres. These differing descriptions are possibly related to intrinsic differences in the studied populations and the influence of important factors such as nutritional status, physical activity levels, comorbidities and biopsied muscle. Skeletal muscle fibre atrophy is also a hallmark of the physiological aging process and mainly seems to affect type II (fast-twitch) fibres (18-20,22-25,31). Alterations in muscle metabolism are also prevalent, with a decrease in the activity of oxidative and glycolytic enzymes being frequently observed with advancing age (19,29,31). Together, these changes will translate into a progressive loss of muscle strength in elderly subjects (32). This phenomenon has important clinical consequences as muscle weakness is a hallmark feature of the presence of frailty in the elderly, which is a state of decreased functional reserve strongly associated with the risk of disability and overall prognosis (12,33).
Aging and nutritional status
Undernutrition is frequent in elderly subjects, independently associated with age (34) and frequently under-diagnosed (35). Physiological anorexia, smoking, the presence of comorbid medical conditions (including COPD), alterations in smell and taste and changes in physical activity with decreased energy requirements are factors that potentially impact nutriment intake in elderly subjects (34,36-40). Importantly, the presence of malnutrition is associated with changes in body composition and the presence of sarcopenia (36), and is directly associated with prognosis in elderly patients (34,35,41,42). As such, malnutrition can be seen as a component of the frailty syndrome (43), with several studies demonstrating that the quality of dietary intake is related to the risk of frailty (44-46).
Alterations in skeletal muscle function and nutritional status in COPD and comparison to physiological aging
Sarcopenia is one of the most recognized extra-pulmonary manifestations of COPD and has been extensively studied (47-53). Although a thorough description of the mechanisms underlying its presence in COPD patients is outside the scope of this text, we need to highlight the main characteristics of skeletal muscle changes associated with COPD in order to contrast them to the aforementioned changes induced by the aging process. Skeletal muscle fiber redistribution in COPD has been well demonstrated, with a consistent and prominent increase in the proportion of type II fibers relative to type I fibers (54-59), associated with an atrophy of the muscle fibers (54) and alterations in enzymatic metabolism activity characterized by increased glycolytic activity, decreased aerobic metabolism (55,60-62) and an impairment in oxidative metabolism that is predominantly observable during and after exercise (63-65). These anomalies will clinically translate into a loss of muscle mass, strength and endurance in patients with COPD, especially in the lower limbs (66), with a significant negative impact on functional capacity, exercise tolerance and overall prognosis (3,4,67-75).
Nutritional depletion and cachexia are also frequently associated with COPD (9,76-79) and are thought to be related to a complex combination of factors that include, among others, the presence of persistent systemic inflammation (80-82), hypoxemia (83) and an increase in resting energy expenditure that is possibly related to increased respiratory work of breathing (82,84). In addition, hormonal derangements such as the decrease in leptin levels observed in patients with COPD may contribute to body wasting (85). Nutritional status and body composition (especially when evaluated using fat-free mass) in patients with COPD are related to the presence of skeletal dysfunction and sarcopenia (79), and, more importantly, to overall prognosis (7-10,86).
The prevalence of COPD increases with age (87), and as such the relative effects of both conditions on skeletal muscles and nutritional status are complex and difficult to untangle. However, the available evidence described above suggests that the relative contribution of aging and COPD to skeletal muscle dysfunction and nutritional depletion are somewhat different in nature, with aging mainly associated with a reduction of fast-twitch muscle fiber proportion and a decrease in nutriment intake related to physiological alterations in energy requirements and anorexia, while COPD is associated with a decrease in slow-twitch muscle fibers and nutritional depletion more closely related to persistent systemic inflammation and increased metabolic requirements. From a clinical point of view, this notion is supported by a large study of elderly COPD patients, in which age and COPD were respectively independently correlated with different aspects of the nutritional status, with age being related to body functionality score, while COPD was associated with body composition status (88). These findings highlight the potential negative synergistic effects of advanced age and COPD on functional capacity and prognosis and the need for an increase in clinical awareness regarding the co-occurrence of these factors.
COPD as a syndrome of accelerated lung aging
The cellular equivalent of aging is senescence, in which cells permanently cease to divide in response to various stimuli (89). The onset of physiological cellular senescence is triggered by the shortening of telomeres, which are repeated sequences of nucleotides (TTAGGG) located at the end of each chromosomes that act as buffers that protect DNA from deterioration during the replicative cycle. Premature senescence can be triggered by other stimuli such as DNA damage, oxidative stress and expression of oncogenes (90,91). The active replenishment of telomeres can be accomplished by telomerase, but this polymerase is absent from somatic cells, leading to a progressive shortening of telomeres with each cell division (92). When a critical length of telomeres is reached, a senescence signal is sent to the cell. The observation that the number of senescent cells increases with age suggests a link between cellular senescence and the physiological aging process (89,93). Increasing evidence support a relationship between the impaired tissue repairing ability induced by cellular senescence and the development of emphysema: an accumulation of senescent cells in the lung of COPD patients compared with smokers without COPD has been demonstrated in humans (94,95), subjects with COPD show shorter telomeres than age-matched controls (96), telomerase deficiency predisposes to COPD (94) and surexpression of anti-aging proteins such as sirtuin 1 protects against cellular senescence and emphysema (97).
Importantly, senescent cells are not metabolically inactive and, on the contrary, generate an inflammatory reaction characterised by the production of pro-inflammatory mediators (interleukine 1, 6, 8, CCL2, TGF-beta and MMPs) (94,98,99) that propagate a persistent inflammatory state. This senescence-associated secretory phenotype (SASP) can induce senescence in adjacent cells (99,100) and could even, if “spilled-over” to the systemic circulation, play a role in the development of the systemic manifestations of COPD. As discussed in the previous section, some evidence suggests a relationship between the presence of skeletal muscle dysfunction and/or nutritional deficiency in COPD and the presence of a systemic, persistent low-grade inflammatory state (80-82,84,85,101-105). Whether the SASP plays a direct role in the mediation of the relationship between the lung disease and the extra-pulmonary manifestations of COPD remains speculative in nature, but provides a novel target for future research.
Comorbidities and skeletal muscular function/nutritional status in patients with COPD
Patients with COPD often present with comorbid conditions, the most frequent of which include cardiovascular diseases, cerebrovascular diseases, anxio-depressive disorders, osteoporosis, cachexia/muscle weakness, lung cancer, CKD (106-110) and several others less frequently considered (111). In a cross-sectional analysis of a large sample of subjects, patients with COPD had a mean of 3.7 comorbidities (including lung disease), compared with 1.8 in age- and sex-matched controls (112). This difference was associated with a two-fold increase in healthcare utilization in patients with COPD in the same study (112). Furthermore, the presence of comorbidities potentiates the negative effects of COPD on functional capacity, quality of life and overall prognosis (3,4,71,74,113).
Among the frequent comorbidities encountered in patients with COPD, some have also been associated with disorders of skeletal muscle function and nutritional status. In particular, CHF and CKD have both been independently associated with anomalies in body composition, nutritional status and skeletal muscle function and composition. The following section will review the main impacts of CHF and CKD on muscle function and nutritional state in order to contrast and compare them with those of COPD.
CHF and skeletal muscle dysfunction
Loss of peripheral muscle strength and endurance is more frequent in patients with CHF than in age-matched controls (74,114,115), and, as in COPD, seems to preferentially affect the lower limb musculature (116). Biopsy studies of the lower limbs muscles in this population have shown that muscle fibre atrophy is frequent and is directed mostly at type II (fast-twitch) fibres (117,118), although a decrease in type 1 cross-sectional area has also been reported (114). Most available data also support the presence of a type I to type II muscle fibre shift redistribution (118-123), as seen in COPD. From a metabolic perspective, subjects with CHF display reduced levels of fatty acid and carbohydrate enzymatic metabolism, while showing increased levels of anaerobic activity (115,117,118,120,124).
Vescovo et al. investigated the relative contribution of deconditioning to the skeletal muscles anomalies observed in CHF by comparing muscle biopsies from patients with CHF to bedridden patients and healthy controls. Muscle atrophy levels were greater in bedridden patients than CHF subjects, but the proportion of types 1 and 2 myosine heavy chains were respectively decreased and increased in CHF, while the opposite was observed in subjects with disuse atrophy. Other have similarly described differences in the skeletal metabolic enzymatic activity patterns of patients with CHF compared with age- and VO2-matched controls, but these effects were only apparent in men (125). These results suggest that disuse alone cannot account for the myopathy observed in CHF, although further studies directly comparing detrained subjects and patients with CHF are required to further understand this relationship, especially given the fact that the control group in the study by Vescovo et al. (patients that had been bedridden for 1 year) may not be representative of the patients with deconditioning encountered in routine clinical practice.
CHF and nutritional status
Weight loss and cachexia are frequent in patients with CHF (126,127) and are likely the result of a combination of neuroendocrine and metabolic factors that induce inadequate dietary intake, excessive nutriment losses or alterations in metabolism (128-130). Pharmacological therapy such as diuretics, digoxin and angiotensin-converting enzyme inhibitors may cause anorexia (128) in these patients, and while intestinal edema inducing satiety and/or a protein-losing gastroenteropathy has often been suggested as a possible cause of anorexia in CHF, clear data supporting this hypothesis are lacking (131,132). In addition, although a certain contribution of anorexia and/or starvation to cardiac cachexia remain possible, the anomalies in body composition in CHF are not compatible with simple starvation, in which weight loss occurs at the expense of fat tissue, in contrast to what is observed in CHF patients (133), where tissue loss is apparent in muscles, fatty tissue and bones.
As in COPD, CHF can be conceptualized as a syndrome of persistent immune activation inducing a state of low-grade systemic inflammation and a preferential shift towards catabolic metabolism induced by increased levels of inflammatory cytokines (128,133,134). In addition, chronic impaired cardiac function is associated with neurohormonal changes that include, among others, an activation of the sympathetic nervous system. In a study comparing the systemic levels of inflammatory and neurohormonal mediators in CHF patients with or without cachexia, plasma levels of norepinephrine, epinephrine, TNF-alpha and cortisol were higher in in the cachectic group, despite similar baseline values of left ventricular ejection fraction and functional class (133). These results highlight the potential role of systemic inflammation and neurohormonal activation in the development in cachexia in patients with CHF, although further studies are required to elucidate the precise mechanisms and directionality of this relationship.
CKD and skeletal muscle dysfunction
CKD is relatively frequent in patients with COPD and may be underrecognized (109,135). Although less studied than in COPD and CHF, anomalies in the skeletal muscles of patients with CKD have also been described, with skeletal muscle weakness being relatively frequently reported, especially in the lower limbs (136-141). Muscle fibre atrophy of the lower limb muscles has also frequently been reported, and preferentially affects type II (rapid-twitch) fibres (137,139,142,143). Studies evaluating muscle metabolic enzymatic activity levels in patients with CKD have been more scarce, but alterations in oxidative and anaerobic metabolism have been reported in these patients in some (140,144,145), but not all, studies (146).
CKD and nutritional status
The catabolic/anabolic balance is disturbed in CKD, especially when end-stage renal failure is present, and its mechanisms are complex and incompletely understood. Among other factors, the metabolic acidosis that is often present in CKD may play an important role in the development of body wasting by promoting protein degradation, especially at the muscle level (147-152). In fact, even small corrections of metabolic acidosis in patients with CKD improve nutritional status and muscle mass, even without nutritional supplementation (153,154).
Acidosis is thought be at the root, and to act synergistically, with others factors promoting protein catabolism (155) or anti-anabolism such as an increase in circulating glucocorticoid levels, insulin resistance, anomalies in growth hormone and leptin serum levels and increased circulating levels of inflammatory cytokines creating a chronic pro-inflammatory state (148,156-159).
Comparison of muscle dysfunction and nutritional status in patients with COPD, CHF and CKD
As reviewed, the alterations observed in the skeletal muscle of patients with CHF and CKD are very similar to those observed in COPD with, broadly speaking, a switch to a fast-twitch fibre phenotype, loss of oxidative capacity, muscle fibre atrophy and loss of strength/endurance. Although some factors contributing to the eventual reaching of this state seem to be overlapping between these conditions (especially the presence of a persistent systemic inflammatory state and anomalies in nutritional status), the initial causative mechanisms for them vary across diagnoses, suggesting that, in practice, they may act synergistically and additively. To our knowledge, however, very few studies have focussed on the relative contribution of COPD and its comorbidities on skeletal muscle function and nutritional status. Hamilton et al. compared skeletal muscle function in a large group of patients according to the presence of cardiac and/or respiratory disease, and showed that patients with concomitant cardiac and respiratory diseases had lower respiratory and lower limb muscle strength than patients with cardiac disease alone, suggesting an additive deleterious effect of respiratory disease in muscle performance in patients with cardiac diseases (74). These results should be interpreted in light of the fact that a certain proportion of patients were classified in the “respiratory impairment” subgroup based on the presence of low forced expiratory volume in 1 second (FEV1) despite normal FEV1/vital capacity ratio, making the true prevalence of COPD in this subgroup unknown.
In a study that investigated the predictive factors of CKD in patients with COPD, the presence of muscle-skeletal disease and hypoalbuminemia was an independent risk factor for the presence of “concealed” CKD (odds ratio 2.73 and 2.98, respectively), suggesting an additive effect of the presence of both COPD and CKD on the prevalence of these anomalies.
Conclusions
The relationship between aging, COPD and its comorbidities on skeletal muscle function and nutritional status is complex, multidirectional and incompletely understood. Despite this, the current body of knowledge allows the identification of various, seemingly partially independent factors related both to the normal aging process and to the independent deleterious effects of chronic diseases on muscle function and body composition. There is a dire need of studies evaluating the relative contribution of each of these factors, and their potential synergistic effects in patients with COPD and advanced age/comorbid conditions, in order to delineate the best course of therapeutic action in this increasingly prevalent population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Guarascio AJ, Ray SM, Finch CK, et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013;5:235-45. [PubMed]
- Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences--clinical impact, mechanisms, and potential for early intervention. COPD 2008;5:235-56. [Crossref] [PubMed]
- Decramer M, Gosselink R, Troosters T, et al. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J 1997;10:417-23. [Crossref] [PubMed]
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996;153:976-80. [Crossref] [PubMed]
- Soler-Cataluna JJ, Sanchez-Sanchez L, Martinez-Garcia MA, et al. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest 2005;128:2108-15. [Crossref] [PubMed]
- Vilaro J, Ramirez-Sarmiento A, Martinez-Llorens JM, et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med 2010;104:1896-902. [Crossref] [PubMed]
- Chailleux E, Laaban JP, Veale D. Prognostic value of nutritional depletion in patients with COPD treated by long-term oxygen therapy: data from the ANTADIR observatory. Chest 2003;123:1460-6. [Crossref] [PubMed]
- Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:1856-61. [Crossref] [PubMed]
- Schols AM, Broekhuizen R, Weling-Scheepers CA, et al. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005;82:53-9. [Crossref] [PubMed]
- Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med 2006;173:79-83. [PubMed]
- Laveneziana P, Palange P. ERS Research Seminar Faculty. Physical activity, nutritional status and systemic inflammation in COPD. Eur Respir J 2012;40:522-9. [Crossref] [PubMed]
- Morley JE. Frailty and sarcopenia in elderly. Wien Klin Wochenschr 2016;128:439-45. [Crossref] [PubMed]
- Roubenoff R, Rall LC. Humoral mediation of changing body composition during aging and chronic inflammation. Nutr Rev 1993;51:1-11. [Crossref] [PubMed]
- Thompson LV. Age-related muscle dysfunction. Exp Gerontol 2009;44:106-11. [Crossref] [PubMed]
- Franssen FM, Wouters EF, Schols AM. The contribution of starvation, deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. Clin Nutr 2002;21:1-14. [Crossref] [PubMed]
- Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab 2015;12:22-6. [PubMed]
- Orlander J, Kiessling KH, Larsson L, et al. Skeletal muscle metabolism and ultrastructure in relation to age in sedentary men. Acta Physiol Scand 1978;104:249-61. [Crossref] [PubMed]
- Scelsi R, Marchetti C, Poggi P. Histochemical and ultrastructural aspects of m. vastus lateralis in sedentary old people (age 65--89 years). Acta Neuropathol 1980;51:99-105. [Crossref] [PubMed]
- Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22--65 years. Acta Physiol Scand 1978;103:31-9. [Crossref] [PubMed]
- Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol 1979;46:451-6. [PubMed]
- Poggi P, Marchetti C, Scelsi R. Automatic morphometric analysis of skeletal muscle fibers in the aging man. Anat Rec 1987;217:30-4. [Crossref] [PubMed]
- Grimby G, Danneskiold-Samsoe B, Hvid K, et al. Morphology and enzymatic capacity in arm and leg muscles in 78-81 year old men and women. Acta Physiol Scand 1982;115:125-34. [Crossref] [PubMed]
- Klitgaard H, Mantoni M, Schiaffino S, et al. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 1990;140:41-54. [Crossref] [PubMed]
- Coggan AR, Spina RJ, King DS, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 1992;47:B71-6. [Crossref] [PubMed]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 1988;84:275-94. [Crossref] [PubMed]
- Chilibeck PD, McCreary CR, Marsh GD, et al. Evaluation of muscle oxidative potential by 31P-MRS during incremental exercise in old and young humans. Eur J Appl Physiol Occup Physiol 1998;78:460-5. [Crossref] [PubMed]
- Chilibeck PD, Paterson DH, Cunningham DA, et al. Muscle capillarization O2 diffusion distance, and VO2 kinetics in old and young individuals. J Appl Physiol (1985) 1997;82:63-9. [Crossref] [PubMed]
- Aniansson A, Grimby G, Hedberg M. Compensatory muscle fiber hypertrophy in elderly men. J Appl Physiol (1985) 1992;73:812-6. [Crossref] [PubMed]
- McCully KK, Fielding RA, Evans WJ, et al. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol (1985) 1993;75:813-9. [Crossref] [PubMed]
- Frontera WR, Hughes VA, Fielding RA, et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985) 2000;88:1321-6. [Crossref] [PubMed]
- Aniansson A, Hedberg M, Henning GB, et al. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve 1986;9:585-91. [Crossref] [PubMed]
- Aoyagi Y, Shephard RJ. Aging and muscle function. Sports Med 1992;14:376-96. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Forster S, Gariballa S. Age as a determinant of nutritional status: a cross sectional study. Nutr J 2005;4:28. [Crossref] [PubMed]
- Constans T, Bacq Y, Brechot JF, et al. Protein-energy malnutrition in elderly medical patients. J Am Geriatr Soc 1992;40:263-8. [Crossref] [PubMed]
- Morley JE. Anorexia, body composition, and ageing. Curr Opin Clin Nutr Metab Care 2001;4:9-13. [Crossref] [PubMed]
- Malafarina V, Uriz-Otano F, Gil-Guerrero L, et al. The anorexia of ageing: physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 2013;74:293-302. [Crossref] [PubMed]
- Gariballa S. Nutrition and older people: special considerations relating to nutrition and ageing. Clin Med (Lond) 2004;4:411-4. [Crossref] [PubMed]
- Chapman IM, MacIntosh CG, Morley JE, et al. The anorexia of ageing. Biogerontology 2002;3:67-71. [Crossref] [PubMed]
- Benelam B. Satiety and the anorexia of ageing. Br J Community Nurs 2009;14:332-5. [Crossref] [PubMed]
- Leslie W, Hankey C. Aging, Nutritional Status and Health. Healthcare (Basel) 2015;3:648-58. [Crossref] [PubMed]
- Payette H, Coulombe C, Boutier V, et al. Weight loss and mortality among free-living frail elders: a prospective study. J Gerontol A Biol Sci Med Sci 1999;54:M440-5. [Crossref] [PubMed]
- Lorenzo-Lopez L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr 2017;17:108. [Crossref] [PubMed]
- Shikany JM, Barrett-Connor E, Ensrud KE, et al. Macronutrients, diet quality, and frailty in older men. J Gerontol A Biol Sci Med Sci 2014;69:695-701. [Crossref] [PubMed]
- Chan R, Leung J, Woo J. Dietary Patterns and Risk of Frailty in Chinese Community-Dwelling Older People in Hong Kong: A Prospective Cohort Study. Nutrients 2015;7:7070-84. [Crossref] [PubMed]
- Bollwein J, Diekmann R, Kaiser MJ, et al. Dietary quality is related to frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2013;68:483-9. [Crossref] [PubMed]
- Gea J, Agustí A, Roca J. Pathophysiology of muscle dysfunction in COPD. Journal of Applied Physiology 2013;114:1222-34. [Crossref] [PubMed]
- Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;189:e15-62. [Crossref] [PubMed]
- Barreiro E. Skeletal Muscle Dysfunction in COPD: Novelties in The Last Decade. Arch Bronconeumol 2017;53:43-4. [PubMed]
- Barreiro E, Gea J. Molecular and biological pathways of skeletal muscle dysfunction in chronic obstructive pulmonary disease. Chron Respir Dis 2016;13:297-311. [Crossref] [PubMed]
- Gea J, Pascual S, Casadevall C, et al. Muscle dysfunction in chronic obstructive pulmonary disease: update on causes and biological findings. J Thorac Dis 2015;7:E418-38. [PubMed]
- Puig-Vilanova E, Martinez-Llorens J, Ausin P, et al. Quadriceps muscle weakness and atrophy are associated with a differential epigenetic profile in advanced COPD. Clin Sci (Lond) 2015;128:905-21. [Crossref] [PubMed]
- Barreiro E, Sznajder JI, Nader GA, et al. Muscle dysfunction in patients with lung diseases: a growing epidemic. Am J Respir Crit Care Med 2015;191:616-9. [Crossref] [PubMed]
- Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 1998;30:1467-74. [Crossref] [PubMed]
- Wang XN, Williams TJ, McKenna MJ, et al. Skeletal muscle oxidative capacity, fiber type, and metabolites after lung transplantation. Am J Respir Crit Care Med 1999;160:57-63. [Crossref] [PubMed]
- Satta A, Migliori GB, Spanevello A, et al. Fibre types in skeletal muscles of chronic obstructive pulmonary disease patients related to respiratory function and exercise tolerance. Eur Respir J 1997;10:2853-60. [Crossref] [PubMed]
- Maltais F, Sullivan MJ, LeBlanc P, et al. Altered expression of myosin heavy chain in the vastus lateralis muscle in patients with COPD. Eur Respir J 1999;13:850-4. [Crossref] [PubMed]
- Jobin J, Maltais F, Doyon JF, et al. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil 1998;18:432-7. [Crossref] [PubMed]
- Jakobsson P, Jorfeldt L, Brundin A. Skeletal muscle metabolites and fibre types in patients with advanced chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respir J 1990;3:192-6. [PubMed]
- Maltais F, Simard AA, Simard C, et al. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 1996;153:288-93. [Crossref] [PubMed]
- Maltais F, LeBlanc P, Whittom F, et al. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax 2000;55:848-53. [Crossref] [PubMed]
- Jakobsson P, Jorfeldt L, Henriksson J. Metabolic enzyme activity in the quadriceps femoris muscle in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;151:374-7. [Crossref] [PubMed]
- Tada H, Kato H, Misawa T, et al. 31P-nuclear magnetic resonance evidence of abnormal skeletal muscle metabolism in patients with chronic lung disease and congestive heart failure. Eur Respir J 1992;5:163-9. [PubMed]
- Payen JF, Wuyam B, Levy P, et al. Muscular metabolism during oxygen supplementation in patients with chronic hypoxemia. Am Rev Respir Dis 1993;147:592-8. [Crossref] [PubMed]
- Kutsuzawa T, Shioya S, Kurita D, et al. 31P-NMR study of skeletal muscle metabolism in patients with chronic respiratory impairment. Am Rev Respir Dis 1992;146:1019-24. [Crossref] [PubMed]
- Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2000;20:353-60. [Crossref] [PubMed]
- Watz H, Waschki B, Meyer T, et al. Physical activity in patients with COPD. Eur Respir J 2009;33:262-72. [Crossref] [PubMed]
- Van Vliet M, Spruit MA, Verleden G, et al. Hypogonadism, quadriceps weakness, and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:1105-11. [Crossref] [PubMed]
- Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010;36:81-8. [Crossref] [PubMed]
- Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:972-7. [Crossref] [PubMed]
- Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:809-13. [Crossref] [PubMed]
- Killian KJ, Summers E, Jones NL, et al. Dyspnea and leg effort during incremental cycle ergometry. Am Rev Respir Dis 1992;145:1339-45. [Crossref] [PubMed]
- Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015;70:213-8. [Crossref] [PubMed]
- Hamilton AL, Killian KJ, Summers E, et al. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med 1995;152:2021-31. [Crossref] [PubMed]
- Barreiro E, Gea J. Respiratory and Limb Muscle Dysfunction in COPD. COPD 2015;12:413-26. [Crossref] [PubMed]
- Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J 2014;44:1504-20. [Crossref] [PubMed]
- Schols AM, Soeters PB, Dingemans AM, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993;147:1151-6. [Crossref]
- Wilson DO, Rogers RM, Hoffman RM. Nutrition and chronic lung disease. Am Rev Respir Dis 1985;132:1347-65. [PubMed]
- Vermeeren MA, Creutzberg EC, Schols AM, et al. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med 2006;100:1349-55. [Crossref] [PubMed]
- Di Francia M, Barbier D, Mege JL, et al. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;150:1453-5. [Crossref] [PubMed]
- Schols AM, Buurman WA, Staal van den Brekel AJ, et al. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996;51:819-24. [Crossref] [PubMed]
- Koehler F, Doehner W, Hoernig S, et al. Anorexia in chronic obstructive pulmonary disease--association to cachexia and hormonal derangement. Int J Cardiol 2007;119:83-9. [Crossref] [PubMed]
- Raguso CA, Luthy C. Nutritional status in chronic obstructive pulmonary disease: role of hypoxia. Nutrition 2011;27:138-43. [Crossref] [PubMed]
- Nguyen LT, Bedu M, Caillaud D, et al. Increased resting energy expenditure is related to plasma TNF-alpha concentration in stable COPD patients. Clin Nutr 1999;18:269-74. [Crossref] [PubMed]
- Schols AM, Creutzberg EC, Buurman WA, et al. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:1220-6. [Crossref] [PubMed]
- Celli BR, Cote CG, Marin JM, et al. The Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in Chronic Obstructive Pulmonary Disease. New England Journal of Medicine 2004;350:1005-12. [Crossref] [PubMed]
- Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998-2009. NCHS Data Brief 2011.1-8. [PubMed]
- Battaglia S, Spatafora M, Paglino G, et al. Ageing and COPD affect different domains of nutritional status: the ECCE study. Eur Respir J 2011;37:1340-5. [Crossref] [PubMed]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell 2007;130:223-33. [Crossref] [PubMed]
- Ben-Porath I, Weinberg RA. When cells get stressed: an integrative view of cellular senescence. J Clin Invest 2004;113:8-13. [Crossref] [PubMed]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol 2005;37:961-76. [Crossref] [PubMed]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005;120:513-22. [Crossref] [PubMed]
- Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 2004;114:1299-307. [Crossref] [PubMed]
- Amsellem V, Gary-Bobo G, Marcos E, et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011;184:1358-66. [Crossref] [PubMed]
- Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 2006;174:886-93. [Crossref] [PubMed]
- Morla M, Busquets X, Pons J, et al. Telomere shortening in smokers with and without COPD. Eur Respir J 2006;27:525-8. [Crossref] [PubMed]
- Yao H, Chung S, Hwang JW, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 2012;122:2032-45. [Crossref] [PubMed]
- Coppe JP, Desprez PY, Krtolica A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5:99-118. [Crossref] [PubMed]
- Dagouassat M, Gagliolo JM, Chrusciel S, et al. The cyclooxygenase-2-prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med 2013;187:703-14. [Crossref] [PubMed]
- Faner R, Rojas M, Macnee W, et al. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2012;186:306-13. [Crossref] [PubMed]
- Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012;7:e37483. [Crossref] [PubMed]
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574-80. [Crossref] [PubMed]
- Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of 'overspill' of inflammatory mediators from the lungs? Review of the evidence. Thorax 2010;65:930-6. [Crossref] [PubMed]
- Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1414-8. [Crossref] [PubMed]
- To M, Swallow EB, Akashi K, et al. Reduced HDAC2 in skeletal muscle of COPD patients. Respir Res 2017;18:99. [Crossref] [PubMed]
- Anecchino C, Rossi E, Fanizza C, et al. Prevalence of chronic obstructive pulmonary disease and pattern of comorbidities in a general population. Int J Chron Obstruct Pulmon Dis 2007;2:567-74. [PubMed]
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009;33:1165-85. [Crossref] [PubMed]
- Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med 2009;122:348-55. [Crossref] [PubMed]
- Incalzi RA, Corsonello A, Pedone C, et al. Chronic renal failure: a neglected comorbidity of COPD. Chest 2010;137:831-7. [Crossref] [PubMed]
- Negewo NA, McDonald VM, Gibson PG. Comorbidity in chronic obstructive pulmonary disease. Respir Investig 2015;53:249-58. [Crossref] [PubMed]
- Milkowska-Dymanowska J, Bialas AJ, Zalewska-Janowska A, et al. Underrecognized comorbidities of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015;10:1331-41. [PubMed]
- Mapel DW, Hurley JS, Frost FJ, et al. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med 2000;160:2653-8. [Crossref] [PubMed]
- Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:155-61. [Crossref] [PubMed]
- Lipkin DP, Jones DA, Round JM, et al. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol 1988;18:187-95. [Crossref] [PubMed]
- Sunnerhagen KS, Cider A, Schaufelberger M, et al. Muscular performance in heart failure. J Card Fail 1998;4:97-104. [Crossref] [PubMed]
- Buller NP, Jones D, Poole-Wilson PA. Direct measurement of skeletal muscle fatigue in patients with chronic heart failure. Br Heart J 1991;65:20-4. [Crossref] [PubMed]
- Mancini DM, Coyle E, Coggan A, et al. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation 1989;80:1338-46. [Crossref] [PubMed]
- Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 1990;81:518-27. [Crossref] [PubMed]
- Drexler H, Riede U, Munzel T, et al. Alterations of skeletal muscle in chronic heart failure. Circulation 1992;85:1751-9. [Crossref] [PubMed]
- Schaufelberger M, Eriksson BO, Grimby G, et al. Skeletal muscle alterations in patients with chronic heart failure. Eur Heart J 1997;18:971-80. [Crossref] [PubMed]
- Sullivan MJ, Duscha BD, Klitgaard H, et al. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc 1997;29:860-6. [Crossref] [PubMed]
- Vescovo G, Volterrani M, Zennaro R, et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart 2000;84:431-7. [Crossref] [PubMed]
- Vescovo G, Serafini F, Facchin L, et al. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart 1996;76:337-43. [Crossref] [PubMed]
- Schaufelberger M, Eriksson BO, Held P, et al. Skeletal muscle metabolism during exercise in patients with chronic heart failure. Heart 1996;76:29-34. [Crossref] [PubMed]
- Duscha BD, Annex BH, Green HJ, et al. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol 2002;39:1170-4. [Crossref] [PubMed]
- Aquilani R, Opasich C, Verri M, et al. Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol 2003;42:1218-23. [Crossref] [PubMed]
- Grossniklaus DA, O'Brien MC, Clark PC, et al. Nutrient intake in heart failure patients. J Cardiovasc Nurs 2008;23:357-63. [Crossref] [PubMed]
- Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest 1999;115:836-47. [Crossref] [PubMed]
- Sanches Machado d'Almeida K, Dalira Schweigert Perry I, Clausell N, et al. Adequacy of energy and nutrient intake in patients with heart failure. Nutr Hosp 2015;31:500-7. [PubMed]
- Sciatti E, Lombardi C, Ravera A, et al. Nutritional Deficiency in Patients with Heart Failure. Nutrients 2016;8:E442. [Crossref] [PubMed]
- Buchanan N, Cane RD, Kinsley R, et al. Gastrointestinal absorption studies in cardiac cachexia. Intensive Care Med 1977;3:89-91. [Crossref] [PubMed]
- King D, Smith ML, Lye M. Gastro-intestinal protein loss in elderly patients with cardiac cachexia. Age Ageing 1996;25:221-3. [Crossref] [PubMed]
- Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 1997;96:526-34. [Crossref] [PubMed]
- Anker SD, Ponikowski PP, Clark AL, et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J 1999;20:683-93. [Crossref] [PubMed]
- Gaddam S, Gunukula SK, Lohr JW, et al. Prevalence of chronic kidney disease in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med 2016;16:158. [Crossref] [PubMed]
- Diesel W, Noakes TD, Swanepoel C, et al. Isokinetic muscle strength predicts maximum exercise tolerance in renal patients on chronic hemodialysis. Am J Kidney Dis 1990;16:109-14. [Crossref] [PubMed]
- Fahal IH, Bell GM, Bone JM, et al. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrol Dial Transplant 1997;12:119-27. [Crossref] [PubMed]
- Kempeneers G, Noakes TD, van Zyl-Smit R, et al. Skeletal muscle limits the exercise tolerance of renal transplant recipients: effects of a graded exercise training program. Am J Kidney Dis 1990;16:57-65. [Crossref] [PubMed]
- Lazaro RP, Kirshner HS. Proximal muscle weakness in uremia. Case reports and review of the literature. Arch Neurol 1980;37:555-8. [Crossref] [PubMed]
- Pastoris O, Aquilani R, Foppa P, et al. Altered muscle energy metabolism in post-absorptive patients with chronic renal failure. Scand J Urol Nephrol 1997;31:281-7. [Crossref] [PubMed]
- Wanic-Kossowska M, Koczocik-Przedpelska J. Myoneuropathy in patients with chronic renal failure treated with hemodialysis (HD) and intermittent peritoneal dialysis (IPD). II. Sensory and motor nerve conduction velocity in patients with chronic renal failure treated with intermittent peritoneal dialysis and hemodialysis. Pol Arch Med Wewn 1996;95:237-44. [PubMed]
- Diesel W, Emms M, Knight BK, et al. Morphologic features of the myopathy associated with chronic renal failure. Am J Kidney Dis 1993;22:677-84. [Crossref] [PubMed]
- Kouidi E, Albani M, Natsis K, et al. The effects of exercise training on muscle atrophy in haemodialysis patients. Nephrol Dial Transplant 1998;13:685-99. [Crossref] [PubMed]
- Conjard A, Ferrier B, Martin M, et al. Effects of chronic renal failure on enzymes of energy metabolism in individual human muscle fibers. J Am Soc Nephrol 1995;6:68-74. [PubMed]
- Durozard D, Pimmel P, Baretto S, et al. 31P NMR spectroscopy investigation of muscle metabolism in hemodialysis patients. Kidney Int 1993;43:885-92. [Crossref] [PubMed]
- Clyne N, Esbjornsson M, Jansson E, et al. Effects of renal failure on skeletal muscle. Nephron 1993;63:395-9. [Crossref] [PubMed]
- Hara Y, May RC, Kelly RA, et al. Acidosis, not azotemia, stimulates branched-chain, amino acid catabolism in uremic rats. Kidney Int 1987;32:808-14. [Crossref] [PubMed]
- May RC, Bailey JL, Mitch WE, et al. Glucocorticoids and acidosis stimulate protein and amino acid catabolism in vivo. Kidney Int 1996;49:679-83. [Crossref] [PubMed]
- Mitch WE. Malnutrition: a frequent misdiagnosis for hemodialysis patients. J Clin Invest 2002;110:437-9. [Crossref] [PubMed]
- Price SR, Mitch WE. Metabolic acidosis and uremic toxicity: protein and amino acid metabolism. Semin Nephrol 1994;14:232-7. [PubMed]
- Williams B, Hattersley J, Layward E, et al. Metabolic acidosis and skeletal muscle adaptation to low protein diets in chronic uremia. Kidney Int 1991;40:779-86. [Crossref] [PubMed]
- Williams B, Layward E, Walls J. Skeletal muscle degradation and nitrogen wasting in rats with chronic metabolic acidosis. Clin Sci (Lond) 1991;80:457-62. [Crossref] [PubMed]
- Pickering WP, Price SR, Bircher G, et al. Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int 2002;61:1286-92. [Crossref] [PubMed]
- Stein A, Moorhouse J, Iles-Smith H, et al. Role of an improvement in acid-base status and nutrition in CAPD patients. Kidney Int 1997;52:1089-95. [Crossref] [PubMed]
- Szeto CC, Chow KM. Metabolic acidosis and malnutrition in dialysis patients. Semin Dial 2004;17:371-5. [Crossref] [PubMed]
- Kalantar-Zadeh K, Mehrotra R, Fouque D, et al. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial 2004;17:455-65. [Crossref] [PubMed]
- Kalantar-Zadeh K, Block G, McAllister CJ, et al. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 2004;80:299-307. [Crossref] [PubMed]
- May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest 1986;77:614-21. [Crossref] [PubMed]
- Mitch WE, Wilcox CS. Disorders of body fluids, sodium and potassium in chronic renal failure. Am J Med 1982;72:536-50. [Crossref] [PubMed]


