Breast carcinoma is a multifactorial disease involving FOXN3, SINA3 and NEAT through repression of GATA3 and TJP
Fox proteins superfamily
Forkhead box (Fox) proteins are a superfamily of transcriptional regulators, which control a wide range of biological processes, therefore, a disturbance of Fox activity can alter cell development, initiate tumorogenesis and cancer metastasis. There are several Fox subfamilies such as FoxN, FoxO, FoxM, FoxP, FoxC and FoxA that are involved in tumor development and progression. Fox proteins can be considered as direct and indirect targets for therapeutic intervention, as well as biomarkers to predict and monitor treatment responses (1).
The regulation of Fox proteins activity is an important process that can be controlled by multiple post-translational modifications as phosphorylation, acetylation and ubiquitylation. These modifications control the site and activity of fox proteins within the cells. Fox proteins in the nucleus are active and act as transcriptional regulators; while Fox proteins in the cytoplasm are inactive and subjected to proteosomal degradation. The alternation in Fox proteins site whether in the nucleus or the cytoplasm is mediated through their import and export from the nucleus. This is regulated through a complex sequence of phosphorylation and acetylation (1).
Functions of Fox proteins
FoxP3 plays a regulatory role in T cell differentiation in addition to cancer initiation and progression (2). It has been reported that FoxP3 expression is down regulated in multiple tumors as breast, ovarian and prostatic carcinoma, which indicates that FoxP3 is an important tumor suppressor gene. In prostate and breast cancer cells, FoxP3 inhibits the expression of SATB1, MYC, HER2 and Skp2 oncogenes and stimulates the expression of P21 and LATS2 tumor suppressor genes so act to inhibit tumor growth and proliferation (3).
Foxp3 recruits other anti-tumor enzymes that inhibit CD39 and CD8. In melanoma and ovarian cancer patients, CD39 is over expressed which protects tumor cells by allowing them to create their “escape phase” in which tumor cells divide rapidly and cannot be detected clinically. Tumor cells create immunosuppressive microenvironment and become independent of the extracellular matrix. As a consequence, the tumor cells completely evade the immune system and metastasize (4).
Nuclear Foxp3 regulates NF-KB and NFAT, transcription factors which regulate cytokine production, transcription of DNA, cell survival and apoptosis. P60 inhibits Foxp3’s translocation to the nucleus thus inhibiting the ability of the cells to induce apoptosis, allowing the cancerous cell to survive and reproduce (4). In addition, FOXN3 acts as a tumor suppressor, inhibiting cell proliferation by down regulating E2F5 (5).
Metastasis is a highly organized process; it is directed by receptors for specific chemotactic cytokines, or chemokines. The most common sites for metastatic spread of breast cancer cells include the lung, liver, brain and bones. These sites are known to express the chemokine CXCL12 that stimulates the receptor CXCR4 which is frequently expressed by breast cancer cells. The interaction between CXCL12 and CXCR4 increases HER2 level (3).
Normal healthy breast epithelium expresses low levels of HER2 as FOXP3 directly binds and represses the expression of ErbB2. ErbB2 increases the expression of HER2. In case of cancer breast, FOXP3 loses the capability to repress ErbB2, thus the levels of ErbB2 transcripts increases, resulting in a greater expression of cell surface HER2 (HER2 is a cell surface molecule and is an important prognostic marker which is overexpressed in breast cancer and correlates to a more aggressive phenotype). The increased expression of HER2 results in the increased expression of CXCR4 which allows more of the CXCR4 ligand and CXCL12 to bind and increase the expression of HER2, thereby creating a feedback loop (3).
FOXP3 is a tumor suppressor gene, it promotes cell adhesion through repression of CD44 protein expressions, leading to reduction of metastatic invasion of breast carcinoma cells (6).
CD44 promotes epithelial mesenchymal transition (EMT) in many cancer types by up regulating mesenchymal markers and down regulating epithelial markers. CD44 inhibits the formation of the membrane-associated E-cadherin-β-catenin complex, which resulted in the nuclear translocation of β-catenin and activation of transcription of genes related to cell invasion and migration (7).
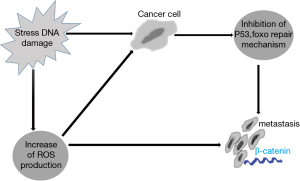
FOXO is also a tumor suppressor gene that inhibits tumorigenesis through senescence induction. FOXO acts to prevent the establishment of damaged cells; such cells are continuously exposed to stress by induction of apoptosis or repair mechanisms (Figure 1). Damaged cells can potentially progress to tumor cells. Many tumors provide a protective mechanism to counter act the tumor suppressor effect of FOXO. These tumors exhibit constitutive PI3K-protein kinase B (PKB) signaling. Enhanced constitutive PI3K-PKB signaling inhibits FOXO signaling and inhibits apoptosis, thus favoring damaged cell establishment (8).
On the other side, tumor progression by itself generates a stressful condition caused by rapid proliferation and decrease oxygen and nutrient supply to these large numbers of cells. In this condition, the oxidative stress can overcome the inhibitory effect of PI3K-PKB caused by tumor cells on FOXO. This means that the tumor cells can use FOXO for its own to withstand these adverse conditions. This can occur by interaction of FOXO and β-catenin, encouraging metastasis to escape from these local adverse conditions. So FOXO can act both as tumor suppressors and as homeostatic regulators in tumor cells (8).
Long non coding RNA NEAT1
Nuclear enriched abundant transcript 1 (NEAT1), is a long non-coding RNA (lncRNA), involved in the development of many cancer types and it is found to be unregulated in breast cancer cell lines and promotes its growth by acting on miR-101 (9).
NEAT1 like other lncRNA cannot be translated to protein, but act to form a subnuclear particle called paraspeckles. Although the function of this particle is still unknown, it seems to play a protective mechanism for cancer cells. When cancer cells are stressed or have DNA damage, P53 up regulates NEAT expression leading to formation of these paraspeckles to allow repair mechanisms. Thus NEAT and paraspeckles are required for cancer cells survival, as the normal cells don’t rely on this mechanism. This is confirmed by the fact that mice lacking NEAT1 are protected from skin cancer development, moreover loss of NEAT1 leads to increased chemosensitivity and cell death. NEAT1 helps cancer cells to grow opportunistically and survive standard chemotherapeutics. Therefore drugs targeting NEAT1 are promising agents in cancer therapy (10).
SIN3A is a repressor transcription factor
SIN3 is a large protein having an important role in regulation of transcription. It has multiple protein–protein interaction domains which act as a scaffold to help in assembly of corepressor complex. The mammalian SIN3A corepressor complex contains 7–10 tightly associated polypeptides, including HDAC1, HDAC2, RbAp46, RbAp48, RBP1, SAP30, and p33ING1b, which associate with SIN3 and act as a component of a “core” SIN3 corepressor complex (11). Sin3A can be considered as a negative regulator of multiple cancer-associated factors as p53, Rb, E2F and Myc (12).
Sin3A interact with DNA-binding or adaptor proteins to target the promoter of the considered gene, as it lacks intrinsic DNA binding domain. Examples for these proteins are: Mad, NRSF, p53 and MeCP2 (13).
Estrogen receptor alpha (ERα) can both activate and repress gene transcription, depending on the ligand, so it can be considered as ligand activated transcription factor. Estrogen can inhibit ERα gene transcription by recruitment of Sin3A to target promoters. In case of estrogen treatment, ERα is recruited to ERα gene (ESR1) on two sites, one at the proximal (A) promoter and the other at distal (ENH1). Coactivator proteins, AIB1 and p300, are recruited at both ERα-binding sites. Whereas Sin3A repressor recruitment, histone modifications and loss of RNA polymerase II, occurred only at the proximal (A) promoter. The repression effect of ERα caused by Sin3A at the proximal (A) promoter of ESR1 overcomes activating factors in distal and proximal regions (14). This can be approved as this estrogen induced inhibition on ESR1is eliminated when the expression of SIN3A is reduced by RNA interference.
Wanjin et al., 2017 reported that FOXN3 can be considered as a transcription repressor. It interacts in human cells with the SIN3A complex. They found that only in ER+ breast cancer cells with absence of RNase, FOXN3 with the SIN3A complex interaction is detected. Depending on the previous studies which revealed that protein-protein interactions requires several lncRNAs (15), they identified, by iRIP-Seq, that lncRNA, NEAT1 is required for the interaction, and proposed that FOXN3 recruits SIN3A complex by the aid of NEAT1 to the chromatin to inhibit the transcription of several gens, suggesting that NEAT1 is important regulator at the epigenetic level.
In addition to the known effect of SIN3A in transcription inhibition, the SIN3A complex has a protein component SAP18; it is an RNA-binding protein that can interact with NEAT1. NEAT1-SIN3A complex can be recruited by multiple transcription repressors other than FOXN3 in other cell types, which means that NEAT1 is a facultative component of the SIN3A complex.
FOXN3-NEAT1-SIN3A complex and breast cancer
Depending on a previous finding that NEAT1 is an ERα target in prostate cancer and in breast carcinoma with ER+ cells where NEAT1 is induced by estrogen (16), Wanjin et al., 2017 reported that by ChIP-Seq studies and by analysis of NEAT1 CHART-Seq results, FOXN3- NEAT1-SIN3A complex inhibit the transcription of GATA3 and TJP1, and both FOXN3 and NEAT1 are involved in malignancies of several tissues through promotion of EMT and invasive metastasis of breast cancer cells (17).
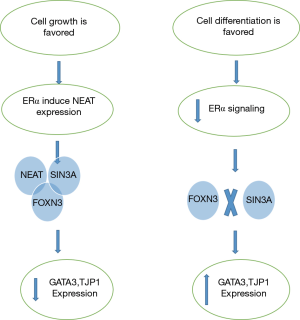
There is a feedback loop interaction between ERα and FOXN3-NEAT1-SIN3A complex. When ERα activates NEAT1, it interacts with SIN3A which then complexed with FOXN3 and form FOXN3-NEAT1-SIN3A complex (Figure 2) (17).
ERα is required for ERα+ breast carcinoma cells growth and multiplication. Since cells growth and differentiation are different and uncoupled process (18), in mammary epithelial cells when growth process is favored, ERα induces NEAT1 to assemble in a complex with SIN3A and FOXN3 and inhibits the transcription of GATA3 and TJP1 genes responsible for maintenance and differentiation. On the other hand when the cell differentiation is favored, the growth signal initiated by ERα is attenuated allowing the expression of GATA3 and TJP1. The balance in this regulatory mechanism is required for normal development of mammary epithelial cells. The disturbance in this balance leads to breast cancer development and metastasis (17).
GATA3 and TJP1 genes and cancer cells metastasis
GATA3 is a transcription factor restricted to mammary epithelium where it is highly expressed. It maintains luminal epithelial cell differentiation (19).
GATA3 is responsible for cell differentiation and inhibition of cancer cell metastasis, so when its expression is lost in breast carcinoma, it indicates bad prognosis. GATA3 produces its effect by inducing microRNA-29b (miR-29b) expression. miR-29b can inhibit metastasis by acting on VEGFA, ANGPTL4, PDGF, LOX and MMP9, ITGA6, ITGB1 and TGFB. These factors act as pro-metastatic regulators by acting on angiogenesis, collagen remodeling and proteolysis, thus miR-29b can affect cell differentiation and epithelial plasticity. Even in GATA3-expressing cells, loss of miR-29b, increases metastasis and promotes a mesenchymal phenotype. GATA3 can also inhibit cancer metastasis by increasing the expression of E-cadherin, increasing cell adhesion. GATA3 and its miR-29b seem to be a new target in cancer therapy (20).
Tight junction protein-1 is a peripheral membrane protein of 220-kD that is encoded by the TJP1 gene. It acts as a scaffold protein to anchor the Tight Junction (TJ) strand proteins, which are fibril-like structures within the cell membrane lipid bilayer, to the actin cytoskeleton (21).
TJPs have an important role in the normal epithelial function. TJPs establish and maintain epithelial cell polarity by acting as a diffusion barrier to proteins and lipids movement through the cell membrane and monitoring multiple processes such as cell polarity, morphogenesis, cell differentiation and proliferation (22).
The impairment of tight junction proteins functions are observed in many cancers. This impairment leads to increase in entry of nutrients and signaling peptides and down-regulation of cell-cell adhesion molecules leading to increased motility and metastasis of cancer cells (23).
Fox proteins and cancer therapy
Since Fox superfamily proteins have important roles in different biological processes, any disturbance in its signaling will lead to development of a wide range of disease, even cancer development and metastasis. Fox proteins can act as oncogenes or tumor suppressor genes through their interaction with regulatory factors as p53 and ER. Thus fox proteins can be considered as a target in breast carcinoma therapy in combination with other treatment strategies.
This combination reduces the toxic side effects of these strategies on normal cells. For example, a chemo-resistant breast cancer cell lines when sensitized to activates FOXO3A, it becomes more responsive to chemotherapy (1).
ER alpha is a prognostic factor for breast carcinoma invasiveness, as ER positive cancer is more responsive to endocrinal treatment than ER negative. Also ER alpha expression is controlled by FOXA1, FOXO3A and FOXM1 (24).
Therapies that target factors controlling Fox expression or its post translational regulation can modify the chemo sensitivity of breast cancer cells. HDACs inhibition modulates FOXO3A activity in cancer cells and induces apoptosis when DNA damage occurs rather than DNA repair and cell cycle arrest. Therefore the fact that the Fox proteins can be considered as novel agents in breast carcinoma therapy requires further investigations (1).
Acknowledgements
Thanks to editorial team of JTD for their manuscript invitation to be published in the Journal of Thoracic Disease.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007;7:847-59. [Crossref] [PubMed]
- Fontenot JD, Rasmussen JP, Williams LM, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005;22:329-41. [Crossref] [PubMed]
- Douglass S, Ali S, Meeson AP, et al. The role of FOXP3 in the development and metastatic spread of breast cancer. Cancer Metastasis Rev 2012;31:843-54. [Crossref] [PubMed]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057-61. [Crossref] [PubMed]
- Sun J, Li H, Huo Q, et al. The transcription factor FOXN3 inhibits cell proliferation by downregulating E2F5 expression in hepatocellular carcinoma cells. Oncotarget 2016;7:43534-45. [PubMed]
- Paulis YW, Huijbers EJ, van der Schaft DW, et al. CD44 enhances tumor aggressiveness by promoting tumor cell plasticity. Oncotarget 2015;6:19634-46. [Crossref] [PubMed]
- Cho SH, Park YS, Kim HJ, et al. CD44 enhances the epithelial-mesenchymal transition in association with colon cancer invasion. Int J Oncol 2012;41:211-8. [PubMed]
- Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 2013;14:83-97. [Crossref] [PubMed]
- Qian K, Liu G, Tang Z, et al. The long non-coding RNA NEAT1 interacted with miR-101 modulates breast cancer growth by targeting EZH2. Arch Biochem Biophys 2017;615:1-9. [Crossref] [PubMed]
- Adriaens C, Standaert L, Barra J, et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med 2016;22:861-8. [Crossref] [PubMed]
- Das TK, Sangodkar J, Negre N, et al. Sin3a acts through a multi-gene module to regulate invasion in Drosophila and human tumors. Oncogene 2013;32:3184-97. [Crossref] [PubMed]
- Zhang Y, Akinmade D, Hamburger AW. The ErbB3 binding protein Ebp1 interacts with Sin3A to repress E2F1 and AR-mediated transcription. Nucleic Acids Res 2005;33:6024-33. [Crossref] [PubMed]
- Roopra A, Sharling L, Wood IC, et al. Transcriptional repression by neuron-restrictive silencer factor is mediated via the Sin3-histone deacetylase complex. Mol Cell Biol 2000;20:2147-57. [Crossref] [PubMed]
- Ellison-Zelski SJ, Solodin NM, Alarid ET. Repression of ESR1 through actions of estrogen receptor alpha and Sin3A at the proximal promoter. Mol Cell Biol 2009;29:4949-58. [Crossref] [PubMed]
- Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010;329:689-93. [Crossref] [PubMed]
- Chakravarty D, Sboner A, Nair SS, et al. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 2014;5:5383. [Crossref] [PubMed]
- Li W, Zhang Z, Liu X, et al. The FOXN3-NEAT1-SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J Clin Invest 2017;127:3421-40. [Crossref] [PubMed]
- Liang J, Shang Y. Estrogen and cancer. Annu Rev Physiol 2013;75:225-40. [Crossref] [PubMed]
- Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 2007;9:201-9. [Crossref] [PubMed]
- Chou J, Lin JH, Brenot A, et al. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat Cell Biol 2013;15:201-13. [Crossref] [PubMed]
- Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochimica et Biophysica Acta (BBA) – Biomembranes 2009;88:872-91. [Crossref]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 2004;286:C1213-28. [Crossref] [PubMed]
- Huo Q, Kinugasa T, Wang L, et al. Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer. Anticancer Res 2009;29:851-7. [PubMed]
- Madureira PA, Varshochi R, Constantinidou D, et al. The Forkhead box M1 protein regulates the transcription of the estrogen receptor alpha in breast cancer cells. J Biol Chem 2006;281:25167-76. [Crossref] [PubMed]