Spontaneous regionalization of esophageal cancer surgery: an analysis of the National Cancer Database
Introduction
Esophagectomy, which represents one of the most effective treatments for esophageal cancer, is a particularly dangerous procedure. Approximately half of esophagectomy patients experience a postoperative complication (1) and up to 14% of patients die as a result of esophageal cancer surgery, making esophagectomy one of the most dangerous cancer surgeries performed (2).
Outcomes after esophagectomy appear to obey a volume-dependent relationship, with high-volume hospitals (HVHs) performing better than low-volume hospitals (LVHs) (3-9). Over the past decade, formal regionalization was implemented in parts of Canada to restrict the performance of esophagectomy to HVHs, reducing mortality rates from 9.6% to 3.6% (10). A similar effort for pancreatic surgery decreased perioperative mortality from 10.4% to 2.2% (11).
There are multiple barriers to formal regionalization of surgical care in countries without centralized health care systems such as the United States (U.S.). However, an increasing awareness by clinicians, patients, and payers of the relationship between hospital attributes and surgical outcomes can lead to a spontaneous movement of care away from LVHs (“spontaneous regionalization”). The Canadian province of Quebec (which did not impose a formal regionalization plan) experienced a similar realignment of complex surgical patients away from LVHs as provinces in which a formal regionalization effort took place (11). In the U.S., health care providers and private payers have become particularly sensitized to volume-outcome relationships (12-14). For example, the Leapfrog Group, a consortium of healthcare purchasers, published a volume threshold for esophagectomy of ≥13 per year to define HVHs (15). The objective of this study was to determine the extent to which the progressive recognition of esophagectomy risk at LVHs in the U.S. and the efforts of private payers have led to a “spontaneous” movement of esophagectomy patients away from LVHs in the U.S. for their care.
Methods
Data source
The National Cancer Database (NCDB) captures approximately 70% of the newly diagnosed cancer patients in the U.S. (16) In addition to detailed demographic, tumor, treatment, and outcomes data, the NCDB allows for hospital-specific surgical volumes to be studied. This study was granted a waiver for patient consent by the Institutional Review Board from the Yale School of Medicine (IRB under HIC Protocol Number 1103008160).
Patient selection
The 2013 NCDB participant user file was queried for all patients >20 years of age, diagnosed with invasive esophageal cancer who underwent esophagectomy between 2004 and 2012. Patients were excluded if they underwent esophagectomy at a hospital different from the hospital that reported their case, or if a total gastrectomy or laryngectomy was listed as part of their resection.
Patients were divided into two eras, 2004–2006 (Era 1) and 2010–2012 (Era 2). These eras were chosen because they represent the periods during and after Leapfrog recommendations were implemented and in which there became increased awareness of the importance of volume-outcome relationships in U.S. hospitals. Additionally, this is the same period in which spontaneous regionalization was observed in the Canadian system.
Hospital volume threshold
Hospital esophagectomy volume during each era was calculated as a 3-year average. The cut point for high and low volume was based on Leapfrog recommendations (15) and hospitals with an average annual volume of ≥13 esophagectomies were considered HVHs, while those with <13 were considered LVHs.
Volume averages were calculated only from years the hospital was reporting (as determined by examining each hospital’s surgical and nonsurgical activity in lung and esophageal cancer). Of the 871 hospitals included in this analysis, 6 hospitals (0.7%) had at least one year that they did not report to the NCDB.
Independent variables
Patient demographic variables analyzed included age, sex, race, ethnicity, income, education level, insurance status, urban/rural designation, and travel distance to the treating hospital. The NCDB uses a modified Charlson-Deyo score to characterize comorbidity burden which stratifies patients to a score of 0, 1, or ≥2. Tumor characteristics included histology, grade, location, size, and pathologic stage. Finally, receipt of neoadjuvant chemotherapy with or without radiation was analyzed. Chemotherapy was defined as multi-agent therapy. Radiation therapy was defined as at least 4,140 cGy (17) directed to the esophagus, chest, or regional lymph nodes. Hospital characteristics analyzed included the type of hospital (academic vs. non-academic), geography, and hospital esophagectomy volume.
Adjusted 90-day mortality
Adjusted 90-day mortality was calculated using the standardized mortality ratio (SMR), defined as the ratio of observed mortality in a given hospital to expected mortality of patients treated at HVHs (18). Expected mortality was estimated using only the subset of patients treated at HVHs, since patient-level risk factors may be confounded by hospital risk at LVHs. Expected mortality was derived from a multivariable logistic regression model, adjusted for patient-level covariates including age, gender, comorbidity score, and tumor characteristics. The model did not adjust for hospital-level data such as academic status and geographic location, as this may obscure the hospital-specific effect and thus the impact of regionalization (19). Regression coefficients from logistic regression were applied to patients treated at LVHs such that each patient was assigned an expected mortality rate. Adjusted 90-day mortality rates were subsequently used to divide patients into risk terciles (e.g., high-risk, mid-risk, and low-risk). While the adjusted 90-day mortality rates were calculated using the SMR (i.e., the ratio of observed to expected mortality), another metric, excess mortality, was defined as the observed mortality beyond what was expected from the risk model (i.e., observed-expected).
Statistical analysis
Bivariate analysis was performed using the χ2 test for categorical variables and nonparametric tests such as the ANOVA Kruskal-Wallis test for continuous variables. Risk differences for categorical variables are available upon request. Missing data were included and coded as “unknown”. All statistical tests were two-sided. A P value <0.05 was considered statistically significant. All statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients and treatment across eras
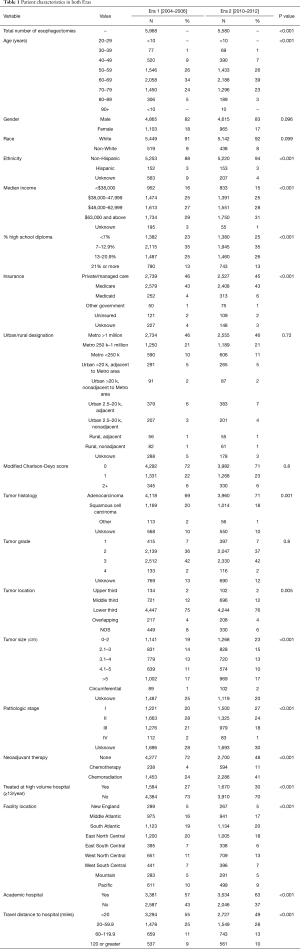
The NCDB captured 5,968 esophagectomy patients in Era 1 [2004–2006], and 5,580 in Era 2 [2010–2012], representing a 6.5% decline between the two eras (P<0.001). Several important differences were noted between the two eras, including a greater prevalence of stage I disease in Era 2 (27% vs. 20%, P<0.001) and a greater use of induction therapy in Era 2 (52% vs. 28%, P<0.001) (Table 1).
Full table
Spontaneous regionalization—patient perspective
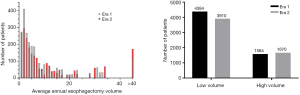
Fewer patients were treated at LVHs in Era 2 (n=3,910) compared to Era 1 (n=4,384, P<0.001), and more patients were treated at HVHs (n=1,670 vs. n=1,584, P<0.001) (Figure S1). More patients were cared for at academic centers in Era 2 (63%, n=3,534) compared to Era 1 (57%, n=3,381, P<0.001). In addition, the proportion of patients that traveled >20 miles increased from 45% (n=2,674) in Era 1 to 51% (2,853, P<0.001) in Era 2.
Spontaneous regionalization—hospital perspective
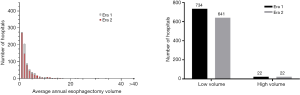
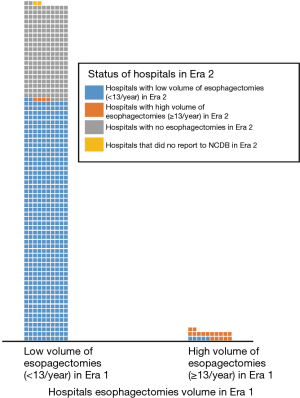
Seven hundred and fifty-six hospitals performed esophagectomies in Era 1 compared to 663 in Era 2, representing a net reduction of 12.4% (P=0.014). Overall, 97% of hospitals in this study were LVHs. Of the 548 hospitals that performed esophagectomies in both eras, 98% (n=539) maintained the same volume status in both eras (Figure 1). Interestingly, all 208 hospitals that stopped performing esophagectomies after Era 1 were LVHs, but 99% of the 115 hospitals that started performing esophagectomies in Era 2 were also LVHs (Table S1). The combined effect resulted in a net decrease in the number of LVHs from Era 1 [734] to Era 2 [641], which did not alter the overwhelming predominance of LVHs in the NCDB (97% in both eras, P=0.7) (Figure S2).

Full table
Quality metrics across eras
Several outcomes measures improved between Eras 1 and 2 (Table 2). Unadjusted 90-day mortality decreased from 10% to 8% (P<0.001). The 90-day SMR improved from 1.38 (95% CI, 1.27–1.49) to 1.14 (95% CI, 1.04–1.25, P=0.002). Median length of stay decreased by 1 day (11 vs. 10, P<0.001). The positive margin rate decreased from 10% to 7% (P<0.001), and the number of lymph nodes removed at the time of surgery increased from 9 to 13 (P<0.001). The 30-day readmission rate was 7% in both eras (P=0.49). A subset analysis of only hospitals that performed esophagectomies during both eras demonstrated similar quality improvements (Table S2).
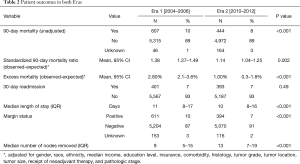
Full table
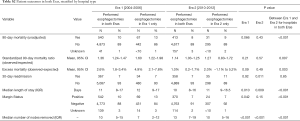
Full table
Relationship between volume status and quality metrics
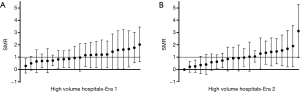
The volume-outcome relationship appeared to vary between eras. In Era 1, patients treated at LVHs had a higher 90-day SMR compared to patients treated at HVHs (SMR 1.50, 95% CI, 1.37–1.63 vs. 1.00, 95% CI, 0.82–1.20; P<0.001) (Table 3). In Era 2, there was a trend toward a higher SMR for LVHs, but it did not reach statistical significance. Substantial variation of the SMR was observed within HVHs in both eras (Figure 2).
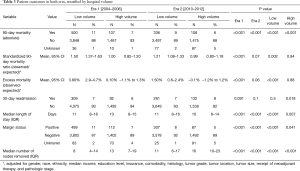
Full table
Impact of hospital attrition on quality metrics
Hospitals that stopped performing esophagectomies after Era 1 (all LVHs) appeared to achieve inferior quality metrics compared to hospitals that continued performing esophagectomies (Table S2). More specifically, hospitals that stopped performing esophagectomies trended toward inferior unadjusted 90-day mortality (13% vs. 10%, P=0.066) compared to hospitals that contributed to both eras, although the SMR was not significantly different. Hospitals that started performing esophagectomies after Era 1 (99% LVHs) had similar unadjusted 90-day mortality and SMR as hospitals that performed esophagectomies in both eras.
Care of the most vulnerable patients
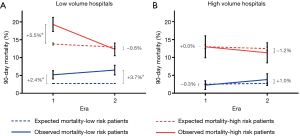
To evaluate the care of the most vulnerable population, patients were stratified by their risk for 90-day mortality. A disproportionately high number of high-risk patients were treated at LVHs (77% in Era 1, P<0.001; 73% in Era 2, P=0.017). Differences in 90-day mortality for high-risk patients between LVHs and HVHs narrowed between Era 1 (19.3% LVH vs.13% HVH, P=0.003) and Era 2 (12.3% LVH vs. 11.3% HVH, P=0.57).
The largest excess mortality was observed when high-risk patients were treated at LVHs, particularly in Era 1 (Figure 3). The magnitude of excess mortality at LVHs varied over time. When high-risk patients were treated at LVHs in Era 1, the excess mortality was 5.5% (95% CI, 3.5–7.5%, P<0.001). When high-risk patients were treated at LVHs in Era 2, no excess mortality was observed (excess mortality −0.6%, 95% CI, −2.4% to 1.1%, P=0.49). On the other hand, excess mortality in low-risk patients treated at LVHs increased over time from 2.4% (95% CI, 1.3–3.6%, P<0.001) to 3.7% (95% CI, 2.4–5.1%, P<0.001).
Discussion
The NCDB paints a mixed picture regarding the extent to which esophagectomy care has been regionalizing spontaneously. More specifically, several trends support the notion that spontaneous regionalization has occurred. For example, over 200 low-volume, poorer performing hospitals stopped performing esophagectomies. This is a key component of regionalization based on the Canadian experience with formal regionalization (10). On the other hand, of the 115 hospitals that started performing esophagectomies, 114 were LVHs. This represents a net decrease in the number of hospitals performing esophagectomy of 12.3%. Our findings are similar to (but not as dramatic as) another study that noted a 20% decrease in hospitals performing esophagectomies in the U.S. during this time period (12). Because of this spontaneous regionalization, the proportion of patients receiving their care at LVHs decreased only slightly (73% to 70%) in our study.
The relationship between annual procedural volume and esophagectomy outcome is similarly complex in the NCDB cohort. When HVHs are defined according to the Leapfrog criteria, patients appear to have better outcomes when treated at HVHs. These findings parallel those of other observational studies that found LVHs to have worse outcomes (3,4,6,7). However, in the current study the volume-associated differences in mortality appeared to dissipate in Era 2 (excess mortality at LVH 3.8% to 1.5%). This may reflect general quality improvements that equally affected all care environments, as all cohorts experienced reductions in perioperative mortality consistent with other published data (12,20). Among these quality improvements may have been better selection, as the number of esophagectomy patients declined by 6.5%. This reduction is more impressive when one considers the incidence of esophageal cancer captured by the NCDB increased by 5.9% during this time. In addition, there was a 279% increase in the use of “local excisions” (e.g., removal by endoscopy) during the study period.
There are some indications that care improved specifically at the contingency of LVHs. This is most noticeable when patients at highest risk for surgical mortality (most vulnerable) were studied. In Era 1, LVHs had the highest excess mortality. While excess mortality is far from a precise reflection of hospital performance, this parameter should reflect any independent risk resulting from care at LVHs. In Era 2, this excess mortality at LVHs was eliminated for the high-risk cohort. One interpretation of this finding is that LVHs became better at caring for high-risk patients (e.g., better patient selection, enhanced care processes, etc.). However, excess mortality at LVHs persisted for low-risk patients which is counterintuitive and suggests the patient risk model may not be as predictive for LVHs. Finally, not all HVHs demonstrated high quality, which is similar to a finding among Leapfrog hospitals which showed over a fivefold variation in 90-day mortality (14).
Given that only 27/871 (3.1%) hospitals performing esophagectomies in the NCDB were HVHs in either era, it is unrealistic to think that all patients could receive their care at HVHs. It is important to focus on the most vulnerable patient population, not only because most operative deaths are identified in this cohort, but also because patient-derived risk could be anticipated and modulated by particularly safe care environments. The current study would suggest that not all LVHs are unsafe, and not all HVHs are safe. Therefore, although there may be potentially meaningful gains in matching the most vulnerable patients with safest care environments, annual volume is a reasonable, but imperfect surrogate for hospital safety.
The current study contains several limitations in addition to those traditionally associated with observational studies (16). Most importantly, the NCDB is not population-based. Although 70% of new cancer diagnoses are captured, these findings may not be entirely reflective of cancer care in the U.S. Specifically, compared to non-CoC-approved hospitals, CoC-approved hospitals (i.e., those that report to the NCDB) are larger, more frequently in urban locations, and have more cancer-related services available to patients (21). Risk modeling for surgical mortality did not include several potentially important health-related data (performance status, pulmonary function, etc.), which may have impacted the extent to which the model accurately adjusted for competing mortality risk.
In conclusion, although fewer LVHs are performing esophagectomies, most patients continue to have surgery at LVHs. While spontaneous regionalization has not occurred on a large scale, subtle shifts in patient allocation have occurred, and LVHs have made improvements in patient outcomes in the absence of realignment. Attempts to increase the efficiency of patient realignment for esophagectomy must take into consideration the progress LVHs have made in caring for or selecting high-risk patients and recognize that annual surgical volume is an imperfect surrogate for hospital safety. Further study is needed to identify optimal alignment of esophagectomy patients and hospitals in order to reduce surgical mortality.
Acknowledgements
The NCDB is a hospital-based tumor registry run jointly by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board from the Yale School of Medicine (IRB under HIC Protocol Number 1103008160), and a waiver for patient consent was granted by the Institutional Review Board.
References
- Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217-22. [Crossref] [PubMed]
- Hu Y, McMurry TL, Stukenborg GJ, et al. Readmission predicts 90-day mortality after esophagectomy: Analysis of Surveillance, Epidemiology, and End Results Registry linked to Medicare outcomes. J Thorac Cardiovasc Surg 2015;150:1254-60. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital Volume and Surgical Mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Dimick JB, Cowan JAJ, Ailawadi G, et al. National variation in operative mortality rates for esophageal resection and the need for quality improvement. Arch Surg 2003;138:1305-9. [Crossref] [PubMed]
- Dimick JB, Pronovost PJ, Cowan JA, et al. Surgical volume and quality of care for esophageal resection: do high-volume hospitals have fewer complications? Ann Thorac Surg 2003;75:337-41. [Crossref] [PubMed]
- Dudley R, Johansen KL, Brand R, et al. Selective referral to high-volume hospitals: Estimating potentially avoidable deaths. JAMA 2000;283:1159-66. [Crossref] [PubMed]
- Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. [Crossref] [PubMed]
- Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg 2007;94:145-61. [Crossref] [PubMed]
- Urbach DR, Bell CM, Austin PC. Differences in operative mortality between high- and low-volume hospitals in Ontario for 5 major surgical procedures: estimating the number of lives potentially saved through regionalization. CMAJ 2003;168:1409-14. [PubMed]
- Finley CJ, Jacks L, Keshavjee S, et al. The Effect of Regionalization on Outcome in Esophagectomy: A Canadian National Study. Ann Thorac Surg 2011;92:485-90. [Crossref] [PubMed]
- Simunovic M, Urbach D, Major D, et al. Assessing the Volume-Outcome Hypothesis and Region-Level Quality Improvement Interventions: Pancreas Cancer Surgery in Two Canadian Provinces. Ann Surg Oncol 2010;17:2537-44. [Crossref] [PubMed]
- Finks JF, Osborne NH, Birkmeyer JD. Trends in Hospital Volume and Operative Mortality for High-Risk Surgery. N Engl J Med 2011;364:2128-37. [Crossref] [PubMed]
- Reames BN, Ghaferi AA, Birkmeyer JD, et al. Hospital volume and operative mortality in the modern era. Ann Surg 2014;260:244. [Crossref] [PubMed]
- Varghese TK Jr, Wood DE, Farjah F, et al. Variation in Esophagectomy Outcomes in Hospitals Meeting Leapfrog Volume Outcome Standards. Ann Thorac Surg 2011;91:1003-9; discussion 1009-10. [Crossref] [PubMed]
- Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery 2004;135:569-75. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research: A review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers, Version 2, 2016.
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J (Clin Res Ed) 1988;296:1313-6. [Crossref] [PubMed]
- Suter LG, Wang C, Araas M, et al. Hospital-level 30-day All-Cause Unplanned Readmission Following Coronary Artery Bypass Graft Surgery: Updated Measure Methodology Report. Centers for Medicare & Medicaid Services (CMS), 2014.
- Dimick JB, Wainess RM, Upchurch GR Jr, et al. National Trends in Outcomes for Esophageal Resection. Ann Thorac Surg 2005;79:212-6. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of Commission on Cancer–Approved and –Nonapproved Hospitals in the United States: Implications for Studies That Use the National Cancer Data Base. J Clin Oncol 2009;27:4177-81. [Crossref] [PubMed]