Anatomy of the atrial septum and interatrial communications
Introduction
Understanding the atrial septum is crucial for all specialists working in congenital heart disease because atrial septal defects (ASDs), account for the second most common congenital heart malformation. ASDs have a worldwide prevalence of 1.65 per 1,000 live births (95% CI: 1.61 to 1.67) with steep increase in detection following the wide spread use of echocardiography in clinical practice (1).
By definition, an ASD is a direct communication between the atrial cavities that allows shunting of blood. These holes can occur in isolation, or in association with other defects, including the most complex forms of congenital heart disease. However, there are variants of interatrial communications that are located outside the area of the true atrial septum. For a better understanding of the anatomy of the various types of holes we review in this article the developmental anatomy of the normal atrial septum, its structural components, and the morphology of the various types of interatrial communications relevant to cardiologists, fetal and paediatric cardiologists, echocardiographers, obstetric sonographers, interventionalists, electrophysiologists and surgeons.
Developmental anatomy
The embryonic heart develops initially as a straight tube with an arterial pole at the superior end and a venous pole at the inferior end, the latter also being the tube inlet. This tube is initially attached to the body, along its length posteriorly by mesocardium but later most of it separates from this mesocardium via a process of looping. The cardiac ventricles develop from the tube; whilst the inferior venous inlet pole expands to form the atria during the looping process. Blood is brought into the right and left sides of the developing atria by large venous channels. These two venous channels develop into the horns of the sinus venosus into which drain the common cardinal veins (ducts of Cuvier) as well as the vitelline veins from the yolk sac, and the umbilical veins from the placenta. The arterial pole, which is the outlet part of the heart tube, also expands by recruitment of extracardiac cells (2).
There are no borders between the primitive atrium and the sinus venosus by the fourth week of gestation. The horns of the sinus venosus develop asymmetrically. The right horn enlarges quickly, whilst the left horn which is the precursor of the coronary sinus, becomes smaller, merging into the developing left atrioventricular junction. The left duct of Cuvier drains into the coronary sinus. Eventually all the systemic venous blood drains to the right part of the primitive atrium, which joins the developing atrioventricular junction at the atrioventricular canal (Figure 1).
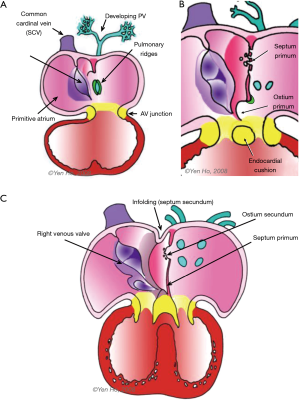
Four endocardial cushions develop to surround the opening of the atrioventricular canal. Two are located laterally, and the other two occupy the superior and inferior borders of the canal. These superior and inferior cushions grow toward each other to meet in the middle thereby dividing the common opening into separate right and left atrioventricular orifices. This process occurs along with septation of the common atrial chamber (3). As discussed below, there are other contributors to septation of the atrioventricular canal.
The posterior aspect of the primitive atrium receives the entire venous component, draining the left and right ducts of Cuvier and the inferior caval vein which forms from the joining of the umbilical and vitelline veins. The atrium moves rightward coincident with its rapid growth and that of the right sinus horn. An anatomical sinuatrial junction becomes recognisable when right and left venous valves develop on each side of the venous component (Figure 1). These valves fuse at their superior and inferior extremities. The septum spurium forms from the continuation of the right venous valve into the posterosuperior wall of the atrium. The Eustachian ridge (sinus septum) forms from the lower portion of the venous valves and is an inward folding of the posteroinferior wall. This fold divides the right venous valve into two portions, remnants of which persist as the Eustachian valve guarding the orifice of the inferior caval vein, and the Thebesian valve guarding entry to the coronary sinus. In between these two valves is a tendinous commissure which extends through the sinus septum. Eventually this forms the anteroinferior rim of the foramen ovale (FO) and an anatomical border for the triangle of Koch which contains the atrioventricular node of the conduction system (3).
The sinus venosus then becomes incorporated into the primitive atrium. The atrial appendages form from growth of the right and left parts of this atrium. In the developed heart, the crista terminalis (terminal crest) demarcates the internal right border between the right atrial appendage and the sinus venosus.
Around the 4th week of gestation, a venous channel forms from an endothelial evagination into the posterior mesocardium behind the heart as the developing lung buds from the trachea. The common pulmonary vein forms from this channel and drains the pulmonary venous plexus into a pit-like orifice. in the posteroinferior part of the primitive atrium (Figure 1) (4).
Atrial septation
Ridges of persisting posterior mesocardium protrude from each side of the orifice of the common pulmonary vein into the atrial cavity (Figure 1). The left ridge is transient whereas the right ridge, also known as the spina vestibuli (vestibular spine), grows bigger and contributes to cardiac septation (3,4). The appearance of the septum primum signifies the onset of atrial septation. It begins as a thin crescent of tissues in the atrial roof. Inferiorly it is continuous with the spina vestibuli. This crescent gradually encroaches into the primitive atrium towards the endocardial cushions (Figure 1). The atrioventricular canal separates into right and left sides by fusion of these cushions and by approximately day 32 the canal enlarges in a rightward direction in order to connect to the precursor of the right ventricle (the distal segment of the looped heart tube). The free edge of the septum primum is covered by mesenchymal cells, derived from embryonic endocardium (5,6). As the septum primum grows toward the atrioventricular junction where the superior and inferior endocardial cushions fuse, the ostium primum which is the space between the atrioventricular junction and the septum primum is gradually obliterated. The ostium disappears by the 6th week. The spina vestibuli becomes muscularised with incorporation of additional mesodermal tissue, and in merging with the septum primum at the atrioventricular junction makes the septum thicker (3). In this process, the inferior ends of the venous valves become displaced anteriorly.
Toward the roof of the partitioning atria, the septum primum disintegrates to form the ostium secondum, allowing blood flow to continue to reach the left heart after septation. Around the 7th week, the common pulmonary vein divides into left and right branches that become incorporated into the posterior wall of the left atrium.
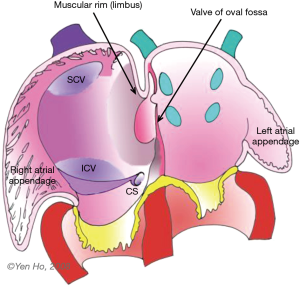
During this time, remodelling occurs in the atrial roof between the septum primum and the superior caval vein (Figure 1). The septum secundum (secondary septum) forms as an infolding of the atrial wall. It folds inward to the right of the septum primum to form the anterosuperior, superior, and posterior margins of the muscular rim (limbus fossa ovalis) of the oval foramen (Figure 2). Thus, the infolded septum secondum is overlapped completely on the left atrial side by the thinner flap-like septum primum that becomes the floor of the oval shaped depression (oval fossa) formed by the infolded rim on the right atrial side (Figure 2). The septum primum functions like a flap valve directing flow from right atrium to left atrium through the ostium secundum which persists as a crucial communication for fetal blood flow. This opening becomes closed after birth when the flap valve, or floor of the oval foramen is pushed against the muscular rim owing to higher pressure in the left atrium. If adhesion is incomplete around the entire margin of the rim, a probe patent foramen ovale (PFO) remains.
Clinical anatomy of the atrial septum
Location
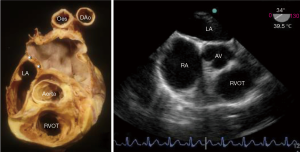
The plane of the atrial septum lies at an angle of approximately 650 from right posterior to left anterior due to the fact that the right atrium is anterior and to the right, whilst the left atrium is posterior and slightly to the left as seen in the 4-chamber transthoracic echocardiogram view and the transoesophageal mid oesophageal aortic valve view approx. 25°–45° (Figure 3). Also of note, this angle increases with pathological left atrial enlargement e.g., with mitral incompetence/stenosis. The septum is seen en-face in the angiographic right anterior oblique view (Figure 4A). Importantly, the antero-superior part of the septal plane is immediately behind the transverse pericardial sinus and the aortic root. Interventional procedures in this part of the septal plane carry a risk of exiting the heart or entering the aortic root.
Furthermore, cardiac interventionists should be aware that, anatomically, the septal structures that directly separate the atrial chambers is considerably less than the entire septal plane depicted on imaging. In other words, the true septum that separates the atrial chambers is limited to the floor of the oval foramen and the immediate margins of the infolded rim around it. Any transgression outside of this area, although in the septal plane, e.g., in performing a transseptal puncture, will cross the right atrial wall into extracardiac tissues before entering the left atrium and is at risk of cardiac tamponade (see below).
Configuration
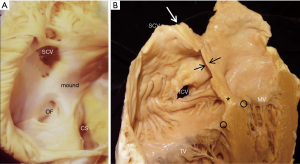
In the fully formed heart, the septal aspect of the right atrium usually has an oval shaped depression (oval fossa) that is surrounded by a raised muscular rim (Figure 4A). Considering that true atrial septum is the part that can be removed without exiting the atrial chambers, the seemingly extensive atrial septum is deceptive because much of the muscular rim is not true septum. In fact, the rim comprises mainly of infolded right atrial wall, which accounts for the peripheral structure superiorly, anterosuperiorly, inferiorly and posteriorly. The septum secundum (the superior and posterior parts of the rim) is mainly the infolded right atrial wall between the base of the superior caval vein and the insertion of the right pulmonary veins to the left atrium (Figure 4B). The wall of the inferior caval vein is continuous with the rim posteroinferiorly. Cardiac surgeons are familiar with this infolding known as Waterston’s (or Sondergaard’s) groove. By dissecting into the epicardial tissues of this interatrial groove or septal raphe, surgeons are able to gain access to the left atrium without entering the right atrium. The sinus node artery is often found in the anterosuperior part of this groove. This small vessel ascends from the right coronary artery to the sinus node at the superior cavo-atrial junction. The groove contains a variable amount of fatty tissue. A false impression of lipomatous hypertrophy of the interatrial septum (LHIS) can be created by excessive amounts of fatty tissue in normal hearts. Echocardiography measurements of fat deposits >1.5 cm in transverse dimension are considered abnormal in young adults (7). LHIS is usually asymptomatic but cases of supraventricular arrhythmia, heart block and sudden death are reported, and misdiagnosis as a tumour may also occur (8). 3D TEE is unable to distinguish this specific arrangement of the atrial wall because fat and wall have similar acoustic impedance and similarly CT cannot because they have similar attenuation values. However, CMR gives exceptionally clear images, because despite water and fat having opposite T1, T2 relaxation times, the T2/T1 ratio of both is similar. CMR balanced SSPP sequence provides a unique T2/T1 weighting, with fat and blood both having high signal. Hence the low signal atrial wall is easily distinguishable between the high signals of blood internally and fat externally (9).
The anteroinferior rim of the oval fossa is continuous with the Eustachian ridge (sinus septum) and the vestibule leading to the tricuspid valve. This area is a “sandwich” (previously known as the muscular atrioventricular septum), consisting of the right atrial wall, fatty tissue of the inferior atrioventricular groove, the wall of the left ventricle and the artery supplying the atrioventricular node of the conduction system. This area separates the right atrium from the left ventricle; due to a disparity in the levels of the hinge lines of the atrioventricular valve at the cardiac septum, with the tricuspid valve attachment being more apical than the mitral valve (Figure 4B).
It should also be noted that related to the antero-superior rim of the fossa is the aortic mound represented by bulging of the right atrial wall into the cavity (Figure 4A). It gives a false impression of being part of the atrial septum. In reality, immediately behind this wall lie the transverse pericardial sinus and the wall of the aortic root.
In fetal life the thin fossa valve is membrane-like comprising of a few myocytes sandwiched between two thin layers of endocardium. Toward birth the valve thickens with increase in muscularity until about 1 year of age. In adulthood endocardial thickening contributes to any increase in thickness.
PFO
The configuration of the normal septum is an oval fossa valve that is sufficiently large to overlap the rim of the fossa. In approximately 25% to 34% of the population, incomplete adhesion between the flap valve and the fossa rim leaves a crevice at the anterosuperior quadrant of the rim that opens to the fetal ostium secundum (Figure 5). At post-mortem this gap called a patent foramen ovale, or PFO, allows a probe to be passed obliquely from the right atrium into the left (10).
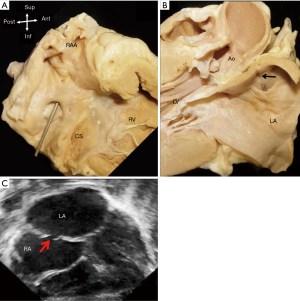
There is considerable confusion regarding the use of the name PFO. Some paediatric cardiologists use the term to describe any central small interatrial communication <4 mm in size seen on echocardiography. It is also sometimes incorrectly used to describe an interatrial communication at the site of the oval fossa valve, or so-called secundum ASD, where the valve is inadequate to overlap the rim so that the foramen ovale (FO) is not obliterated.
In autopsy specimens, there is wide variation in the size of probe-PFO (1–10 mm in diameter) (10,11). The length of the tunnel through which a probe can be passed varies with the extent of overlapping between the flap valve and the rim (11,12). Anatomically, there are two types of PFO. One is valve-competent forming a perfect seal because the valve overlaps the muscular rim without any gaps; thereby preventing any shunting of blood between the atria under normal physiological conditions. Some cases have aneurysmal valves which can bow into either atrium during respiration (Figure 6). The other form of PFO is valve-incompetent due to inadequate overlap between the rim and the valve. This may be secondary to retraction of the aneurysmal valve and/or to atrial dilatation causing stretching of the muscular rim which causes the flap valve to bow into the atrial chambers, decreasing the overlap. This form of PFO may be due to a defect in the rim or may be a secundum defect (a true oval fossa defect) due to inadequate valve tissue. The valve viewed from the left atrial aspect is characterless, other than a few crevices and pits, blending into the atrial wall. However, the crescent-like valve free edge marks the defect (Figure 5). In situations where a wire or catheter is passed through a PFO e.g., when performing a balloon atrial septostomy in a neonate with transposition of the great arteries, it is important for interventionists to beware that there is a danger of perforating the thin anterior wall of the left atrium. The wire tip is guided directly at this weak area, immediately inferior to the Bachmann’s bundle, by the crescent like free edge of the septum primum (13).
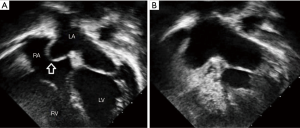
Occasionally, the oval foramen [or FO] closes during intrauterine life. This may lead to massive hypertrophy of the right atrium and ventricle with arrhythmia, right heart failure and congestion, pericardial effusion, pleural effusion, ascites, and nonimmune hydrops and underdevelopment of the left side of heart. Death usually occurs shortly after birth. Such premature closure or oval foramen restriction can be diagnosed in fetal echocardiography using the criteria of a FO diameter <2 mm with a Doppler velocity >120 cm/s or a FO diameter <3 mm with a Doppler velocity–measured gradient >5 mm Hg (14) Directional Enhanced Flow Imaging has recently been reported as useful for early diagnosis for obstetric sonographers (15).
ASDs and interatrial communications
Although a true ASD is a hole in the confines of the atrial septum, the generally used nomenclature is to describe any hole which permits shunting of blood between atrial chambers as an ASD, particularly those greater than 3-4mm in size. Most common are holes known as “secundum defects” that are in the oval fossa. Interatrial communications that outside the true septum include “ostium primum” defects, superior and inferior sinus venosus defects and coronary sinus defects and confluent or common atrium (16) (Figure 7A).
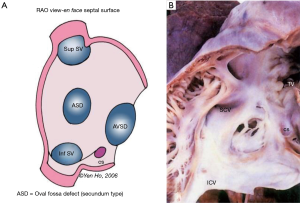
The morphology of ASDs was initially described by Rokitansky (17). Genetics plays a role and there are associations of certain ASD types with particular syndromes. Skeletal abnormalities of the forearm and hand are associated with secundum defects from mutations in TBX5. Familial forms of secondum ASD have been associated with prolonged conduction times and GATA4 and NKX2.5. At the Royal Brompton Hospital in 250 consecutive cases undergoing secondum ASD closure, there was a 2% incidence of family history in 1 or more with close relatives. Although many ASDs are diagnosed in childhood, a significant number first present in adulthood. At our institution in the past 3 years more than 50% presented in adult life.
Secundum defects are holes in the true atrial septum i.e., ASDs. They are located in the flap valve covering the oval fossa, the “septum primum”. Termed secundum defects, alluding to persistence of the embryonic “ostium secundum”, the defects are often larger than the embryonic communication and involve deficiency of the valve itself. It is therefore more accurate to term such defects oval fossa defects. They vary in size from small perforations to complete absence of the valve guarding the oval fossa. There is consensus that ASDs causing right ventricular dilatation should be closed. Such defects are usually ≥10 mm in diameter and occupy at least one third of the length of the total interatrial septum on the four chamber echocardiographic view and cause a Qp:Qs >1.5:1 on MRI flow assessment (18). Of note, spontaneous closure of a small secundum ASD can occur in the first 2 years of life and conversely defects can also enlarge with somatic growth in later childhood and adolescence. The easiest types for transcatheter closure are those defects with complete muscular borders, formed from the valve being too small to overlap the muscular rim, creating an oval-shaped gap. Multiple holes or fenestrations give the valve a mesh like appearance or it may persist as a delicate lace-like remnant (Figure 7B). A typical windsock appearance can be seen on echocardiography when the valve forms an aneurysm. A septal aneurysm is not a contraindication to device closure as long as the left atrial disc can cover most of the septum (18).
In the presence of a secondum ASD the sinus and atrioventricular nodes of the conduction system are in the expected position. Nevertheless, there are other atrial structures in the vicinity to be taken into consideration when deciding how to close these defects. Eccentrically located ASDs and/or very large defects decrease the distances between the defect margins not only to the atrioventricular node, but also the orifice of the coronary sinus, mitral valve, aortic root and the right pulmonary veins. In the most extreme form, the defect may be so large as to leave only a narrow rim around it.
Sinus venosus defects
These defects are located outside the confines of the true septum, superiorly or inferiorly and referred to as superior and inferior sinus venosus defects respectively. Located above the superior rim of the oval fossa, the superior sinus venosus defect allows the orifice of the caval vein to override the septum and drain into both atrial chambers (Figure 8). Consequently, the defect has a well-defined inferior border which is the infolded fossa rim enclosing epicardial fatty tissues, but it has no proper superior border since the orifice of the superior caval vein is above the defect. Usually there is associated partial anomalous connection of the right superior pulmonary vein to the superior caval vein and surgical closure of this defect with a patch should take care to avoid obstructing pulmonary venous return to the left atrium. In hearts with this defect the sinus node is normally sited. Should there be a need to widen the cavo-atrial junction, care should be taken not to damage the sinus node or its arterial supply.
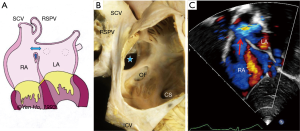
The inferior sinus venosus defect is related to the orifice of the inferior caval vein (Figure 7A). It is far less common than the superior sinus venosus defect but has similar features such as the lack of a complete muscular border. The right inferior pulmonary vein may be connected anomalously to the inferior caval vein. Some cases may be difficult to distinguish from an oval fossa defect that extends toward the inferior caval vein and has a vestigial inferior rim.
Coronary sinus defects
There are many morphologic variations to this group of defects that allow interatrial communication. They are holes at the site of the coronary sinus orifice that allow direct interatrial communication (Figure 7A), or are located on the postero-inferior wall of the left atrium allowing passage of blood between the left atrium and the coronary sinus channel. This entity was previously described as a spectrum of severity due to ‘unroofing of the coronary sinus’ (19) Anatomically speaking, they are holes due to deficiency of not only the wall of the coronary sinus but also the inferior wall of the left atrium that overlies the venous channel. Holes in this location can be large or small, single or multiple. When associated with persistent patency of the left superior caval vein there is direct venous drainage into the left atrium. In the setting of a large defect at the orifice of the coronary sinus, the atrioventricular node may be situated close to the antero-superior margin.
Ostium primum defects
These defects represent persistence of the embryonic ostium primum. It permits interatrial shunting outside of the true atrial septum (Figure 7A). In many cases, the oval fossa is well formed and intact. The hole lies between the free margin of the atrial septum and the atrial surface of the conjoined leaflets of the common atrioventricular valve existing in hearts with atrioventricular septal defects. These defects are better considered within atrioventricular septal defects since these hearts bear the unifying morphologic hallmark of a common atrioventricular junction. Suffice to say, the atrioventricular node in these hearts are abnormally located (20).
Conclusions
Knowledge of the unexpectedly complex nature of the atrial septum, differentiating the true atrial septum from its surroundings, whilst noting its proximity to important nearby structures is advantageous to clinicians promoting the avoidance of complications when closing ASDs or performing transseptal procedures. Distinction between true ASDs and interatrial communications helps in understanding the morphology of holes permitting shunting between the atrial chambers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide. A systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. [PubMed]
- Gittenberger-de Groot A, Bartelings MM, Deruitter MC, et al. Basics of cardiac development for understanding of congenital heart malformations. Pediatr Res 2005;57:169-76. [Crossref] [PubMed]
- Kim JS, Virágh S, Moorman AF, et al. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ Res 2001;88:395-402. [Crossref] [PubMed]
- Lamers WH, Moorman AF. Cardiac septation. A late contribution of the embryonic primary myocardium to heart morphogenesis. Circ Res 2002;91:93-103. [Crossref] [PubMed]
- Mommersteeg MT, Soufan AT, de Lange FJ, et al. Two distinct pools of mesenchyme contribute to the development of the atrial septum. Circ Res 2006;99:351-3. [Crossref] [PubMed]
- Arrechedera H, Alvarez M, Strauss M, et al. Origin of mesenchymal tissue in the septum primum: a structural and ultrastructural study. J Mol Cell Cardiol 1987;19:641-51. [Crossref] [PubMed]
- Shirani J, Roberts WC. Clinical, electrocardiographic and morphologic features of massive fatty deposits ("lipomatous hypertrophy") in the atrial septum. J Am Coll Cardiol 1993;22:226-38. [Crossref] [PubMed]
- Sef D, Turina MI. Septectomy and biatrial resection for extensive septal lipomatosis. J Card Surg 2016;31:683-5. [Crossref] [PubMed]
- Faletra FF, Muzzarelli S, Dequarti MC. Imaging-based right-atrial anatomy by computed tomography, magnetic resonance imaging, and three-dimensional transoesophageal echocardiography: correlations with anatomic specimens. Eur Heart J Cardiovasc Imaging 2013;14:1123-31. [Crossref] [PubMed]
- Ho SY, McCarthy KP, Rigby ML. Morphological features pertinent to interventional closure of patent oval foramen. J Interv Cardiol 2003;16:33-8. [Crossref] [PubMed]
- Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17-20. [Crossref] [PubMed]
- Marshall AC, Lock JE. Structural and compliant anatomy of the patent foramen ovale in patients undergoing transcatheter closure. Am Heart J 2000;140:303-7. [Crossref] [PubMed]
- Ho SY. Embryology and Anatomy of the Atrial Septum. In: Thakur R, editor. Transseptal Catheterization and Interventions. 1st ed, Cardiotext; 2010:11-26.
- Gupta U, Abdulla RI, Bokowski J. Benign outcome of pulmonary hypertension in neonates with a restrictive patent foramen ovale versus result for neonates with an unrestrictive patent foramen ovale. Pediatr Cardiol 2011;32:972-6. [Crossref] [PubMed]
- Li YD, Li ZA, He YH. Premature closure or restriction of the foramen ovale: prenatal diagnosis by directional enhanced flow imaging. J Ultrasound Med. 2013;32:1291-4. [Crossref] [PubMed]
- Ho S, McCarthy KP, Josen M, et al. Anatomic-echocardiographic correlates: an introduction to normal and congenitally malformed hearts. Heart 2001;86:ii3-11. [PubMed]
- Davies MK, Hollman A. Karl Freiherr von Rokitansky (1804-78). Heart 1997;78:425. [Crossref] [PubMed]
- Liegeois-Radojevic J, Rigby ML. Atrial Septal Defect (Interatrial Communication). In: M Gatzoulis, G Webb, P Daubeney, editors. Diagnosis and Management of Adult Congenital Heart Disease 3rd Edition. London: Elsevier, 2017:306-15.
- Raghib G, Ruttenberg HD, Anderson RC, et al. Termination of left superior vena cava in left atrium, atrial septal defect, and absence of coronary sinus; a development complex. Circulation 1965;31:906-18. [Crossref] [PubMed]
- Thiene G, Wenink AC, Frescura C, et al. Surgical anatomy and pathology of the conduction tissues in atrioventricular defects. J Thorac Cardiovasc Surg 1981;82:928-37. [PubMed]


