Expression pattern of soluble triggering receptor expressed on myeloid cells-1 in mice with Acinetobacter baumannii colonization and infection in the lung
Introduction
Acinetobacter baumannii (A. baumannii) is a pleomorphic aerobic gram-negative bacillus exclusively isolated from the hospital and hospitalized people. A. baumannii has low virulence and motility, but it is capable of causing pulmonary infections in vulnerable people with compromised immune function. Currently A. baumannii has emerged as one of the most common and troublesome opportunistic bacterial pathogens in hospital-acquired pneumonia (HAP) (1,2). The carriage ratio of A. baumannii in patients from an intensive care unit is approximately 75%. Even in healthy humans with normal immune systems, its carriage ratio is as high as 25% (3). Due to its multi-drug resistance, there is no clearly effective therapy for HAP caused by A. baumannii (4), which therefore results in results in a mortality rate of 52% (1).
Bacterial colonization, defined as the presence of bacteria on the body surface including the airway without causing clinical evidence of infection in the individual, is the prerequisite of A. baumannii infection (5). A. baumannii colonization does not necessarily lead to pulmonary infection, but it does increase the risk of infection (6). It is difficult to tell whether A. baumannii observed in the culture of respiratory tract samples is infection or colonization (7), especially in patients with multiple co-morbidities, concurrent infections, and who are on prolonged courses of antibiotics. Procalcitonin (PCT) and C-reactive protein (CRP) are commonly used serum biomarkers for diagnosing infections. CRP has relatively low specificity and serum CRP level can be increased in a number of other conditions including trauma, tumor, and autoimmune diseases (8). Compared with CRP, serum PCT concentration has a better diagnostic value due to its high specificity and early response to bacterial infection (9). However, reliable serum biomarkers for distinguishing between A. baumannii infection and colonization have been barely reported so far.
Triggering receptor expressed on myeloid cells-1 (TREM-1) is an immunoglobulin expressed on the surface of myeloid cells that was discovered by Bouchon et al. in 2000 (10). Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) is the soluble form of TREM-1. Increasing numbers of studies have reported that sTREM-1 is secreted into the blood and other body fluids when bacterial infections occur and sTREM-1 levels are closely correlated with the severity of infection (11,12). However, whether sTREM-1 expression levels are different between A. baumannii infection and colonization in the lung has been unknown so far.
In this study, we established a murine model of A. baumannii colonization and infection, and investigated the expression pattern of sTREM-1 in mice with A. baumannii infection or colonization. The correlation between serum sTREM-1 and PCT or CRP concentrations were further analyzed.
Methods
Reagents
Streptozotocin and sodium citrate buffer were purchased from Sigma (St. Louis, MO, USA). Mouse/Rat TREM-1 Quantikine ELISA Kit and Mouse C-Reactive Protein/CRP Quantikine ELISA Kit were obtained from R&D Systems (Minneapolis, MN, USA). Mouse PCT ELISA kit was obtained from USCN (Wuhan, Hubei, China).
Animals
This study was approved by the Institutional Review Board of Central South University and carried out in strict accordance with the guidelines approved by the Animal Care and Use Committee of Central South University. Specific-pathogen-free C57BL/6J male mice (4–6 weeks of age) were purchased from the Center of Laboratory Animals, Central South University (Changsha, Hunan, China). Animals were housed in a specific-pathogen-free laminar-flow atmosphere under controlled temperature, humidity and light with a standard rodent chow diet. Animal experiments were performed after the mice had been acclimated for 1 week.
Experimental design
Mice were randomly divided into a diabetic group (n=150) and a non-diabetic group (n=20). Mice in the diabetic group were injected with 1% streptozotocin (70 mg per kg of body weight) intraperitoneally for 5 days (once per day), while mice in the non-diabetic group were injected with the same volume of sodium citrate buffer (0.1 M, pH =4.5). Tail vein blood glucose was measured in all mice after 10 days, and mice with random blood glucose higher than 16.7 mM were considered to be diabetic. Subsequently, diabetic mice were randomly divided into control, colonization, and infection groups (n=50). Using an ultrasonic atomizer (Rochester, NY, USA), mice in the colonization and infection groups inhaled a suspension solution of A. baumannii at concentrations of 1×109 CFU/mL and 1×1011 CFU/mL, respectively, for 1 h while mice in the control group inhaled saline. Inhalation doses of A. baumannii were chosen based on previous studies (13,14). These mice were euthanized and sacrificed on Days 0, 1, 3, 5 and 7 (n=10 at each time point, mice on Day 0 were sacrificed immediately after A. baumannii inhalation). Blood and pharyngeal swab samples were collected directly after euthanasia. Blood was centrifuged at 3,000 rpm for 5 min to obtain serum samples. For 5 mice in each group, the right lung of each animal was dissected for homogenate culture and the left lung for H&E staining. For the other 5 mice in each group, whole lung homogenate was performed as previously described (15). Briefly, whole lung tissue from each animal was prepared by homogenization in PBS-containing protease inhibitors (Complete, Roche Diagnostics). Lung homogenate was subsequently centrifuged at 12,000 rpm for 10 min at 4 °C, and the supernatant was collected.
H&E staining
Tissue from the left lung was submerged and fixed in 4% paraformaldehyde at 4 °C for 24–48 hours and embedded in paraffin. Sections (4 µm) were stained with hematoxylin and eosin, and the morphological changes of the lungs were observed under a light microscope (Leica Microsystems, Wetzlar, Germany).
Pharyngeal swab and lung homogenate culture
Pharyngeal swabs were collected by gently swabbing the palatine arches and pharynx of each mouse and dissolved in pathogen-free saline. Tissue from the right lung was weighed, minced, immersed in pathogen-free saline, and homogenized at 5,000 rpm for 20 min. After centrifugation at 3,000 rpm for 3 min, the supernatant was collected and diluted 10, 100, 1,000 and 10,000 fold with saline. 100 µL of each sample was evenly added onto blood agar plates (3 plates for each sample). Agar plates were inverted and put into a constant temperature incubator (37 °C) for 24 h. A. baumannii colonies were confirmed by the Department of Clinical Laboratory, Xiangya Hospital (Changsha, Hunan, China). The number of colonies for each sample was subsequently counted and the logarithmic value of bacteria number per gram of lung tissue weight from each mouse was calculated.
Measurement of serum sTREM-1, PCT and CRP
sTREM-1, PCT and CRP concentrations in serum and lung supernatants were determined using commercially available ELISA kits according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed with the Statistical Package for the Social Sciences version 13.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation (SD), and comparisons between groups were evaluated using an independent t-test. Pearson correlation analysis was performed to evaluate the correlation between serum sTREM-1 and PCT or CRP concentrations in mice with A. baumannii inhalation. A P value <0.05 was considered to be statistically significant.
Results
Presentation of A. baumanni infection in mice
After A. baumannii inhalation, the mice in the infection group appeared to have decreased food intake and physical activity and body weight loss (Table 1). Some mice in the infection group displayed signs of conjunctivitis. In contrast, mice in the non-diabetic, control, and colonization groups were overall healthy and did not present any of these symptoms.

Full table
Confirmation of A. baumannii colonization and infection
A. baumannii colonies grew from pharyngeal swab and lung homogenate samples from both the colonization and infection groups, while no colonies were observed in the non-diabetic and control groups. In the colonization group, the colony number of lung homogenate samples was maximal on Day 0 and gradually decreased on subsequent days. However, the colony numbers in the infection group displayed a rapid increase during the first 3 days, peaked on Day 5, and maintained at a stable level for the last 2 days (Table 2).
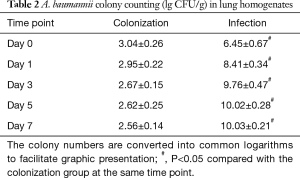
Full table
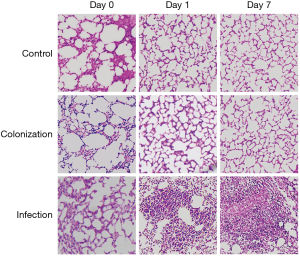
Consistently, histology examination showed that only mild pulmonary edema was seen in lung tissue from control and colonization group mice on Day 0, evidence of which was no longer apparent on the following day. In contrast, lung tissue from infection group mice presented with inflammatory cell infiltration, destruction of alveolar structure, and exudation in the alveoli (Figure 1). Taken together, these data confirmed that the murine model of A. baumannii colonization and infection used in this study was successful.
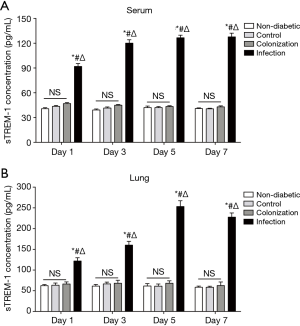
sTREM-1 levels in serum and lung supernatants were elevated after A. baumannii infection but not colonization in the lung
As shown in Figure 2, consistent with A. baumannii colony counts, sTREM-1 concentration also showed a quick and substantial elevation from Day 1 to Day 5, and remained overall stable during the next 2 days, suggesting that the production of sTREM-1 was correlated with development of A. baumannii infection in lung. There was no statistically significant difference in sTREM-1 levels in serum or lung supernatants among non-diabetic, control, and colonization groups. Interestingly, in the infection group, sTREM-1 concentrations in serum and lung supernatants were significantly elevated, approximately to 2-fold compared with control or colonization groups (Figure 2).
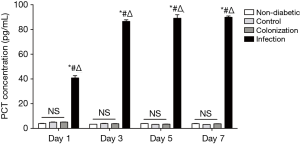
Serum levels of sTREM-1 were positively correlated with PCT and CRP
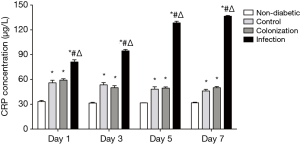
To further assess the diagnostic value of serum sTREM-1 in A. baumannii infection, we investigated the correlation between serum levels of sTREM-1 and PCT or CRP. A significant increase of PCT and CRP in serum was observed after A. baumannii infection (Figures 3,4). There was no significant difference in PCT concentrations among non-diabetic, control, and colonization groups. However, compared with the non-diabetic group, CRP concentrations were significantly higher in control and colonization groups, but no difference was seen between control and colonization groups.
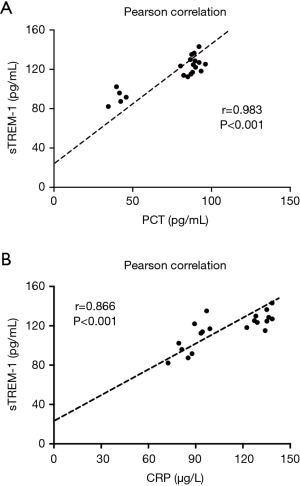
Importantly, Pearson correlation analysis revealed that the elevated serum level of sTREM-1 during the infection time course was positively correlated with PCT (r=0.983, P<0.001) and CRP (r=0.866, P<0.001) (Figure 5), suggesting that like PCT and CRP, sTREM-1 may be used as a serum biomarker for A. baumannii infection.
Discussion
A. baumannii is the most common pathogen that colonizes the body surfaces of patients and health care workers. In the last decade, A. baumannii has become one of the main opportunistic pathogens in HAP. Early and accurate diagnosis of A. baumannii infection in the lung is of fundamental importance for effective therapies. In the present study, we found that the serum level of sTREM-1 was markedly elevated after A. baumannii infection but not colonization in a diabetic murine model. Furthermore, the changes of sTREM-1 concentration after infection positively correlated with serum levels of PCT and CRP.
Distinguishing infection from colonization remains a major challenge for the diagnosis of A. baumannii, especially when patients do not present clear or characteristic symptoms. A mistaken decision will either cause improper use of antibiotics and severe antibiotic resistance, or put patients in a risky situation because of delayed antibacterial treatment. Valuable biomarkers or techniques that could facilitate accurate diagnosis of A. baumannii infection are therefore urgently needed. The prerequisite for exploring potential biomarkers is the establishment of the animal model of A. baumannii infection and colonization. A. baumannii has relatively weak invasiveness and is not capable of infecting animals with a normal immune system. Previously, cyclophosphamide has commonly been used to generate animals with compromised immunity (16). However, most neutrophils are killed in this animal model and lethal infection develops within a short time frame, which makes it difficult to observe the dynamic pathological changes after infection. It has been reported that patients with diabetes are at significantly increased risk for the acquisition of A. baumannii infection (17). Thus, we used a murine model with streptozotocin-induced diabetes for A. baumannii infection based on based on previous studies (14,18-20). A. baumannii colonization and infection were successfully induced by using different doses of inhaled bacteria. This inhalational model of A. baumannii in diabetic mice provides a simple and effective approach to study A. baumannii colonization and infection, thus offering an alternative to existing models for future studies. Mild pulmonary edema and infiltration of inflammatory cells was seen in mice from the control and colonization groups directly after aerosol inhalation and disappeared the next day, which we thought was caused by the non-infectious irritation during inhalation.
TREM-1 is a receptor that triggers an inflammatory cascade and is highly expressed in neutrophils and monocytes (21). It has been reported that activation of TREM-1 immediately induces rapid degranulation of neutrophilic granules and drives the release of pro-inflammatory factors, including MIP-1, MCP-1 and IL-8, thus playing an important regulatory role in inflammation (22). sTREM-1, the soluble form of TREM-1, has been demonstrated to be produced and secreted into body fluids including blood during microbial infections, and its expression level is closely associated with the severity of infection, including HAP caused by A. baumannii (11,23). It is worth noting that the specificity of sTREM-1 in bacterial infection still remains controversial. Bouchon et al. reported that sTREM-1 was not upregulated in samples from patients with non-infectious inflammatory conditions like psoriasis, ulcerative colitis, or immune-complex mediated vasculitis, indicating that this receptor is specifically involved in infection (24). However, increasing amount of studies has shown that serum sTREM-1 is also significantly elevated in noninfectious inflammatory diseases (25-27). Phua et al. observed a significant elevation of serum sTREM-1 levels in patients with COPD and asthma exacerbations compared with control objects, although this elevation was greater in patients with A. baumannii associated pneumonia (28). In the present study, an elevation of sTREM-1 levels in serum was observed after A. baumannii infection, trending similarly to the increase of bacterial colonies. More importantly, we found that similar to PCT, which has been reported to be a biomarker to differentiate A. baumannii infection from colonization (29), there was a significant difference in sTREM-1 levels in both serum and lung supernatants between the colonization and infection groups. The serum level of CRP increased in mice of both control and colonization groups, which may be associated with the inflammatory counterpart of diabetes. A positive correlation between sTREM-1 and PCT or CRP further supports that serum sTREM-1 may also be used as a biomarker in A. baumannii infection. However, whether the combined detection of sTREM-1, PCT, and CRP in serum could promote the diagnostic accuracy of A. baumannii-associated pneumonia is still unexplored.
There are several limitations in the present study. Firstly, data in the present study is obtained exclusively by using murine models instead of humans, which limit the interpretation of potential diagnostic value of sTREM-1 in A. baumannii infection. Furthermore, whether the elevation of sTREM-1 levels is correlated with the severity of A. baumannii infection remains undetermined. Future studies investigating sTREM-1 kinetics in human subjects with A. baumannii associated pneumonia and sepsis are therefore required to address these limitations.
In conclusion, we demonstrated that serum levels of sTREM-1 were upregulated in A. baumannii infection while it remained unchanged at the colonization stage. Furthermore, in A. baumannii-infected mice, sTREM-1 concentrations in serum were positively correlated with PCT and CRP. Collectively, these findings indicate that sTREM-1 may be a promising biomarker for differential diagnosis of A. baumannii colonization and infection in the lung.
Acknowledgements
The authors are grateful to Dr. Margo Emont (University of Michigan, MI, USA) for her generous assistance in language checking, and to Dr. Shichuan Zhang (Central South University, China) for his generous assistance in statistical analysis. The authors also highly appreciate the Department of Clinical Laboratory, Xiangya Hospital, for their assistance in Acinetobacter baumannii identification.
Funding: This study was supported in part by grants from the Scientific Research Foundation of Health and Family Planning Commission of Hunan Province (132015-012), National Natural Science Foundation of China (81600025) and the National Key National Natural Science Foundation of China and Development Program of China (2016YFC1304300).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Central South University and conducted in accordance with the guidelines approved by the ethics committee.
References
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008;21:538-82. [Crossref] [PubMed]
- He R, Luo B, Hu C, et al. Differences in distribution and drug sensitivity of pathogens in lower respiratory tract infections between general wards and RICU. J Thorac Dis 2014;6:1403-10. [PubMed]
- Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 2014;71:292-301. [Crossref] [PubMed]
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 2007;5:939-51. [Crossref] [PubMed]
- Gordon NC, Wareham DW. Evaluation of CHROMagar Acinetobacter for detection of enteric carriage of multidrug-resistant Acinetobacter baumannii in samples from critically ill patients. J Clin Microbiol 2009;47:2249-51. [Crossref] [PubMed]
- Jung JY, Park MS, Kim SE, et al. Risk factors for multi-drug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect Dis 2010;10:228. [Crossref] [PubMed]
- Casadevall A, Pirofski LA. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun 2000;68:6511-8. [Crossref] [PubMed]
- Porfyridis I, Plachouras D, Karagianni V, et al. Diagnostic value of triggering receptor expressed on myeloid cells-1 and C-reactive protein for patients with lung infiltrates: an observational study. BMC Infect Dis 2010;10:286. [Crossref] [PubMed]
- Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin Infect Dis 2004;39:206-17. [Crossref] [PubMed]
- Bouchon A, Dietrich J, Colonna M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 2000;164:4991-5. [Crossref] [PubMed]
- Gibot S, Cravoisy A. Soluble form of the triggering receptor expressed on myeloid cells-1 as a marker of microbial infection. Clin Med Res 2004;2:181-7. [Crossref] [PubMed]
- Gibot S, Kolopp-Sarda MN, Béné MC, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med 2004;200:1419-26. [Crossref] [PubMed]
- Chiang SR, Chuang YC, Tang HJ, et al. Intratracheal colistin sulfate for BALB/c mice with early pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Crit Care Med 2009;37:2590-5. [Crossref] [PubMed]
- Luo G, Spellberg B, Gebremariam T, et al. Diabetic murine models for Acinetobacter baumannii infection. J Antimicrob Chemother 2012;67:1439-45. [Crossref] [PubMed]
- Hu X, Li X, Hu C, et al. Respiratory Syncytial Virus Exacerbates OVA-mediated asthma in mice through C5a-C5aR regulating CD4(+)T cells Immune Responses. Sci Rep 2017;7:15207. [Crossref] [PubMed]
- Manepalli S, Gandhi JA, Ekhar VV, et al. Characterization of a cyclophosphamide-induced murine model of immunosuppression to study Acinetobacter baumannii pathogenesis. J Med Microbiol 2013;62:1747-54. [Crossref] [PubMed]
- Alsultan AA, Hamouda A, Evans BA, et al. Acinetobacter baumannii: emergence of four strains with novel bla(OXA-51-like) genes in patients with diabetes mellitus. J Chemother 2009;21:290-5. [Crossref] [PubMed]
- Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 1976;193:415-7. [Crossref] [PubMed]
- Sheppard DC, Rieg G, Chiang LY, et al. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2004;48:1908-11. [Crossref] [PubMed]
- Li H, Tan H, Hu Y, et al. Small protein A and phospholipase D immunization serves a protective role in a mouse pneumonia model of Acinetobacter baumannii infection. Mol Med Rep 2017;16:1071-8. [Crossref] [PubMed]
- Liu T, Zhou Y, Li P, et al. Blocking triggering receptor expressed on myeloid cells-1 attenuates lipopolysaccharide-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Sci Rep 2016;6:39473. [Crossref] [PubMed]
- Radsak MP, Salih HR, Rammensee HG, et al. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J Immunol 2004;172:4956-63. [Crossref] [PubMed]
- Routsi C, Giamarellos-Bourboulis EJ, Antonopoulou A, et al. Does soluble triggering receptor expressed on myeloid cells-1 play any role in the pathogenesis of septic shock? Clin Exp Immunol 2005;142:62-7. [Crossref] [PubMed]
- Bouchon A, Facchetti F, Weigand MA, et al. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001;410:1103-7. [Crossref] [PubMed]
- Bingold TM, Pullmann B, Sartorius S, et al. Soluble triggering receptor on myeloid cells-1 is expressed in the course of non-infectious inflammation after traumatic lung contusion: a prospective cohort study. Crit Care 2011;15:R115. [Crossref] [PubMed]
- Choi ST, Kang EJ, Ha YJ, et al. Levels of plasma-soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) are correlated with disease activity in rheumatoid arthritis. J Rheumatol 2012;39:933-8. [Crossref] [PubMed]
- Peng L, Zhou Y, Dong L, et al. TGF-beta1 Upregulates the Expression of Triggering Receptor Expressed on Myeloid Cells 1 in Murine Lungs. Sci Rep 2016;6:18946. [Crossref] [PubMed]
- Phua J, Koay ES, Zhang D, et al. Soluble triggering receptor expressed on myeloid cells-1 in acute respiratory infections. Eur Respir J 2006;28:695-702. [Crossref] [PubMed]
- Gao J, Zou Y, Wang Y, et al. Breath analysis for noninvasively differentiating Acinetobacter baumannii ventilator-associated pneumonia from its respiratory tract colonization of ventilated patients. J Breath Res 2016;10:027102. [Crossref] [PubMed]


