Clinical results of multimodality therapy for esophageal cancer with distant metastasis
Introduction
Esophageal cancer is a fatal disease, with especially poor outcomes for advanced stages (1). The median survival time (MST) of stage IV patients receiving chemotherapy is reportedly <1 year (2). The 3-year survival rate of patients with esophageal cancer and distant organ metastasis was previously reported to be approximately 0.3% (3). For 8.6% of patients, the disease is observed to be already spread to other organs at the time of diagnosis (4). Distant metastases occur in the lung, liver, bone and widespread nodal metastases (5). Most patients with metastatic disease experience dysphagia associated with obstruction caused by a large primary lesion. For patients with metastatic esophageal cancer, long-term relief of dysphagia is one of the most important issues regarding their quality of life (QOL) (6). Thus, the purpose of treatment of patients with distant metastasis is to improve overall survival (OS) and remove dysphagia. To rapidly relieve dysphagia, a stent is typically used. However, radiotherapy (RT) after stent placement may cause severe adverse events (perforation), so further treatment becomes difficult once a stent has been used. RT alone is not used as a first-line therapy for management of metastatic esophageal cancer, although it may be used for some patients with obstructions caused by large primary lesion (5). Palliative chemotherapy has been proposed for treatment of metastatic disease, with the aims of controlling the primary lesion and improving QOL. Response rates to chemotherapy alone have ranged from approximately 20% to 40% for metastatic disease (7-9). With respect to palliative concurrent chemoradiotherapy (CCRT) in patients with distant metastasis, previous studies have shown considerable improvement of dysphagia (10-12). In CCRT, the radiation dose has been controversial and may not achieve the recommended dose. At our institution, some patients have received preoperative CCRT plus surgery of the local esophagus and have responded better than patients who received other treatments. However, this experience has not been well analyzed, so it is difficult to identify which patients would benefit from multimodality therapy, including surgery. Although several studies have investigated prognostic factors for patients with localized esophageal cancer only (13), there is limited data on treatment of patients with distant metastasis. Previously, several studies reported results from irradiation (40 and >60 vs. ≤60 Gy) of patients with distant metastases, but these studies did not determine the optimum dose (14,15). Furthermore, the effectiveness of the combination of multidisciplinary treatment and surgery for these patients was not examined. The aim of this retrospective study was to assess the optimal dose for local response in patients with histologically confirmed esophageal squamous cell cancer and distant metastasis and determine if multimodality therapy, including surgery, is beneficial for these patients.
Methods
Patients
This retrospective study included 34 patients (25 males) aged 42–92 years (median, 70 years) with histologically confirmed esophageal squamous cell cancer with distant metastasis who underwent at least one cycle of chemotherapy and RT between January 2005 and December 2016. No patients were diagnosed with adenocarcinoma. All patients had no other active malignant tumor during treatment. All patients were treated at Nihon University Itabashi Hospital. To perform CCRT, the patients were required to meet the following criteria: Eastern Cooperative Oncology Group performance status (PS) 0 or 1, white blood cell count >4,000/µL, neutrophil count >2,000/µL; platelet count >100,000/µL, and 24-hour creatinine clearance >60 mL/min. Patients who died or stopped CCRT immediately because of general worsening of their condition were excluded.
Staging investigations
Staging was performed by using findings of contrast-enhanced computed tomography (CT), gastrointestinal fiberscopy, and barium esophagography. Whole-body CT was performed to examine distant metastases. We defined distant lymph node metastasis as a lymph node with a short-axis diameter of >5 mm, a lymph node with an infiltrative margin, and presence of central necrosis. Positron emission tomography (PET) was performed to identify metastases when CT findings were inconclusive. However, all patients did not undergo PET, especially those treated between 2005 and 2012. We sometimes encounter false-positive PET findings. We compared lymph node size before and after CCRT, and if the lymph node size decreased after CCRT, we interpreted that as evidence of metastasis. Bronchoscopy, bone scintigraphy, and magnetic resonance imaging (MRI) were optionally performed according to the primary and metastatic conditions. Staging was performed according to the UICC’s TNM (6th edition) classification of malignant tumor and distant metastasis was subdivided into M1a/M1b according to the relative location of non-regional lymph nodes and the primary tumor. M1a arises from two circumstances: cervical nodes from upper third cancer or celiac node metastases from lower third cancer. Staging, therapeutic effect, and presence or absence of recurrence were determined by radiologists, radiotherapists, and surgeons.
Chemotherapy and RT
The treatment plan included CT scans, which were assessed in 5-mm sections. The gross tumor volume (GTV) is the volume of the area occupied by the tumor as measured by CT imaging and fiberscopy. The GTV was defined according to the primary tumor and involved node on CT (>0.5 cm along the short axis). The clinical target volume (CTV-primary) for the primary lesion was defined as the GTV + 0.7 cm in the horizontal direction and +5 cm in the craniocaudal direction. The CTV-lymph node for lymph node metastasis was defined as the regional lymph node area +0.5 cm. When the primary lesion existed in the upper esophagus, the CTV-lymph node included the bilateral supraclavicular area as a T-shaped pattern. When the primary lesion existed in the lower esophagus, the CTV-lymph node included the abdominal lesion. If the patient’s general condition was poor, the supraclavicular and abdominal areas were omitted and only the primary area was treated. The internal target volume was defined as the CTV plus a 0.5-cm margin to establish the planning target volume (PTV). The radiation field was defined as the PTV plus a 5-mm leaf margin. A 10-MV X-ray beam was exposed through anterior and posterior opposed portals and an oblique opposed portal. Each beam was created by adjusting the PTV with a margin along the path of the beam. Intensity-modulated RT was not used because our institution had no established policy for use of this treatment modality in patients with esophageal cancer. The Clarkson algorithm was used to calculate the irradiation dose. The minimum and maximum doses according to the PTV were 95% and 107%, respectively. RT was delivered 5 days a week by using a single daily fraction of 1.8 or 2.0 Gy. None of the patients was administered accelerated hyperfractionated RT. After a total dose of 40 Gy, all patients were evaluated clinically by CT, and RT was performed in oblique opposed fields to exclude the cord. After 40 Gy, the RT was performed only for the primary tumor with a 3-cm craniocaudal margin. Basic total radiation doses were 50 Gy because of the palliative intent. Preoperative CCRT was stopped after 40 Gy, and then the patients received surgery.
Chemotherapy comprised protracted infusion of 5-fluorouracil (5-FU) combined with cisplatin (CDDP) with adequate hydration and antiemetic coverage (FP regimen). In general, the patients were treated with 5-FU 700 mg/m2 on days 1–4 and CDDP 70 mg/m2 on day 1. Two cycles of chemotherapy were administered every 4 weeks during RT. Doses were modified according to the judgment of the attending physician: the doses of 5-FU and CDDP generally did not cause hematological adverse events except for leukocytopenia or non-hematological Gr 3 toxicity. All patients stopped CCRT at 30 Gy and were not treated for 7–14 days until their general and hematological conditions recovered. Every effort was made to continue CCRT on schedule. Subcutaneous granulocyte colony-stimulating factor (G-CSF) 150 µg/day was injected if the neutrophil count was 1,000/µL after CCRT. If the patient could not maintain oral intake because of dysphagia, intravenous hyperalimentation was performed to maintain the patient’s nutritional condition. We also performed chemotherapy for recurrences. There was no strict protocol, and FP or TS-1 mainly were given according to the patient’s general condition.
Operation
A small subset of patients also underwent surgery after neoadjuvant CCRT. There was no strict protocol, but our criteria for surgery after CCRT was no organ metastasis, only non-regional lymph node metastasis that was not aggressive before CCRT, and no evidence of tracheoesophageal fistula. When lung nodules could not be identified as metastases because of small nodules, we operated. We stopped RT at 40 Gy in case of surgery. The types of surgical procedures included right-side transthoracic esophagectomy and reconstructed esophagus post sternum. Several-field lymph node dissection was performed for selected patients. Some patients received total gastrectomy because the primary tumor was present in the abdominal esophagus (Ae) area. Salvage esophagectomy was not performed after CCRT.
Evaluation of initial clinical response and toxicity on follow-up
All patients were closely observed during CCRT. Symptom responses were assessed every week during CCRT. After CCRT, the responses were assessed within 1 to 3 months. Patients who had undergone surgery had their symptom responses assessed immediately before the operation. Complete response (CR) was defined as the ability to eat solid and liquid diets without symptoms. Partial response (PR) was defined as the ability to eat a solid diet with some dysphagia or to eat a semi-solid diet. Stable disease (SD) was defined as no change in symptoms before CCRT. Progressive disease (PD) was defined as worsened symptoms before CCRT. It is difficult to distinguish disease symptoms from RT side effects, so symptom responses were scored when the treatment was most effective and side effects disappeared. Follow-up objective assessment of local responses was performed about 4 weeks from completion of treatment by CT or by barium esophagography or gastrointestinal fiberscopy. These scans were performed about 6 months after the first follow-up. The recommended follow-up protocol at our institution includes investigation at 3-month intervals for the first 6 months and every 6 months thereafter. However, this treatment mainly had a palliative intent because many patients who received CCRT died in the short term or changed hospitals and were unable to visit our hospital. Therefore, we were unable to perform strict follow-up for some patients. In these cases, information on the patient’s condition was obtained from the family by telephone. The clinical definitions of CR, PR, SD, and PD were based on the standard definitions established by the World Health Organization (16). Objective assessment of local responses was performed when the treatment was most effective. CR was tentatively defined upon endoscopic observation of the entire esophagus as disappearance of the tumor lesion and absence of cancer cells in biopsy specimens. When gastrointestinal fiberscopy was not performed, we defined CR as disappearance of the primary site on CT findings. When surgery was performed, we defined the local response on the basis of the pathological analysis results. Local recurrence was defined as an increase in tumor size on CT or gastrointestinal fiberscopy. The date of recurrence was determined as the first day when the local recurrence was observed. Adverse events were recorded once per week during CCRT and 4 weeks after treatment according to the Common Terminology Criteria (CTC) for Adverse Events, version 4.0, with toxicity graded as mild (CTC grade 1), moderate (CTC grade 2), severe (CTC grade 3), or life-threatening (CTC grade 4) (17).
Statistical analysis
The OS after CCRT was calculated according to the Kaplan-Meier method and was based on the interval between the last day of treatment and date of death or most recent follow-up as of 2016. Data from patients who reached the end of the follow up period without sustaining an event were censored. Local control (LC) was calculated on the basis of the interval from the last day of treatment until local recurrence. Data from patients who died with no evidence of recurrence were censored. Univariate survival comparisons were performed by using the log-rank test. The analyzed prognostic factors for overall survival were age (<70 vs. ≥70 years), sex (male vs. female), distant organ metastasis (yes vs. no), objective response (<CR vs. CR), receipt of surgery (yes vs. no), and radiation dose (≤50 vs. >50 Gy). Independent variables that showed a statistically significant association on univariate analysis were included in the multivariate analysis. Fisher exact tests were used to compare differences in the symptoms and objective responses relative to the dose of RT between the groups. P values of <0.05 were considered as indicating statistical significance. All calculations and survival displays were performed by using SPSS 15.0 J statistical software (SSPS, Chicago, IL, USA).
Patient consent
This retrospective study analyzed data on diagnosis and treatment. All procedures performed were in accordance with the ethical standards of the institutional review board and national research committees and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patient records/information was anonymized and de-identified prior to analysis in this study. Written patient’s consent to participate can be waived by institutional review board because of retrospective study. We informed all patients at the start of treatment that their treatment data may be used in future research, even if the treatment outcome was death. All patients agreed and consented to the use of their data for future research. We informed merit and demerit of the chemoradiotherapy and operation and consent was obtained from all patients included in the study.
Results
Patient characteristics
The baseline patient characteristics are listed in Table 1. The median age at diagnosis for the entire group was 70 years. Fifteen (44%) patients had positive non-regional lymph nodes only, 19 (56%) had metastases to distant organs, and 8 (23.5%) had more than two distant metastases. If the UICC 7th edition is applied, there were 31 patients classified as M1 because 3 patients had abdominal LN metastasis, which was not classified as M1 in the 7th edition.

Full table
Treatment
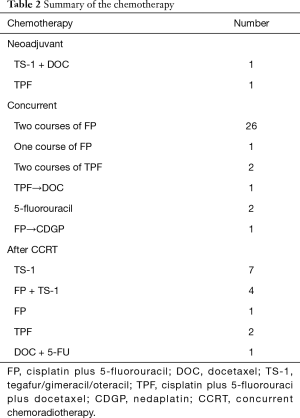
According to our policy, RT should be stopped after the patient receives 30 Gy and suspended for 7–14 days until the patient’s condition improves. Therefore, all patients completed the planned CCRT after the RT suspension interval. Table 2 summarizes the chemotherapy regimen. For two patients, the second cycle of chemotherapy was reduced or changed because of toxicities observed after the first course. One patient received only the first FP chemotherapy. Eight patients received surgery. Seven patients had non-regional lymph node metastases. Only one patient who underwent surgery had a small lung metastasis. Six patients received right-side transthoracic esophagectomies and reconstructed esophagus post sternum. Two patients received total gastrectomy because the primary tumor was located in the Ae area.
Full table
Additional treatment
When local recurrence or new distant metastasis occurred, chemotherapy was restarted. Twelve patients received FP and/or TS-1 if appropriate depending on their general condition. Two patients received neoadjuvant therapy because of a large primary lesion to reduce the radiation field.
Survival
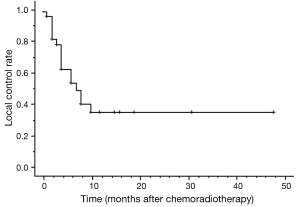
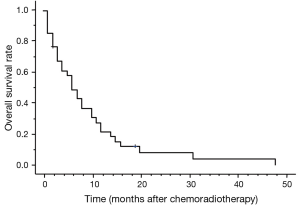
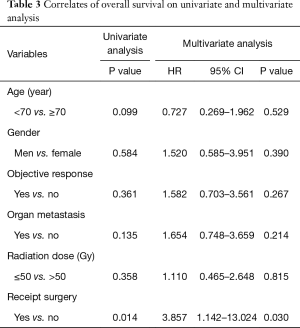
Thirty-one patients died during follow-up. The causes of death were primary disease or related to primary disease in 20 patients, double cancers in 1 patient, and other disease in 1 patient. Even if there was a local effect, most patients died due to disease recurrence. The MST was 5 months. The 1-year OS was 20.6% (Figure 1). The prognostic factors identified on the univariate and multivariate analyses are given in Table 3. Improved OS was associated with receipt of surgery [hazard ratio (HR), 3.857; 95% CI, 1.142–13.024; P=0.030] on both univariate and multivariate analyses, and the MST was 11 months.
Full table
Objective response
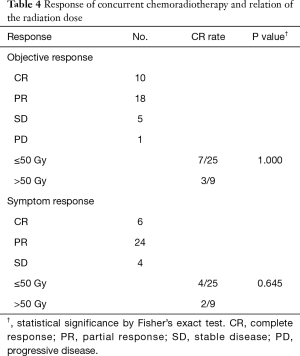
The responses of the primary lesions are shown in Table 4. Objective responses were assessable in 28 patients by gastrointestinal fiberscopy. Four patients did not undergo gastrointestinal fiberscopy and were assessed by CT. Two patients did not undergo either gastrointestinal fiberscopy or CT, so objective response was assessed by barium esophagography. The overall objective response rate was 82% (28/34). Ten patients had CR, 18 had PR. The 1-year LC rate was 35.9% (Figure 2). CR was observed at all doses. All PD patients had tumor progression in the radiation fields. One patient with T4 disease had CR at the primary site. According to the pathological findings after surgery, three patients had CR and five patients had residual tumors. There was no significant difference in the objective response between ≤50 and >50 Gy (Table 4).
Full table
Symptom response
The symptom responses are shown in Table 4. Thirty (88%) patients had CRs (n=6) and PRs (n=24). Four (12%) patients had SD. There was no significant difference in the symptom response between ≤50 and >50 Gy (Table 4).
Toxicity
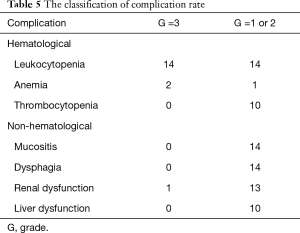
Table 5 shows the toxicities observed in the study population. Fourteen patients had G3 leukocytopenia that was cured by G-CSF treatment. Two patients had G3 anemia that was cured by blood transfusion. One patient had G3 renal dysfunction that was cured by intravenous drip. No treatment-related deaths occurred. Leukocytopenia, acute mucositis, dysphagia, and renal dysfunction were the second most common adverse reactions and were classified as ≤ G2 in 14 (41%) patients.
Full table
Discussion
In this study, the efficacy and safety of CCRT using 5-FU and CDDP with approximately 50 Gy of RT were assessed in patients with distant metastasis to develop more effective treatments. The toxicity was tolerable, and the median OS was 5 months in the patients with distant metastasis. A small group of patients who received surgery (those who did not have distant organ metastasis) also seemed to derive significant benefits in terms of OS. However, most patients at this stage had dysphagia, and such patients are often considered candidates for palliative intent. There was no significant difference in the responses between ≤50 and >50 Gy RT.
Chemotherapy alone can improve QOL and dysphagia in approximately 70% of patients with stage IV disease and provide median OS times of approximately 10 months (8,18). However, survival of patients who had distant metastasis treated with multimodality therapy has been reported to be significantly better than that of patients who received single-modality chemotherapy or best supportive care alone (19). Lee et al. reported that the 1-year OS rates after CCRT were superior to those after palliative chemotherapy alone (45% vs. 18%) in 67 patients with inoperable stage IV esophageal cancer (20). In Japan, Ohtsu et al. were the first to perform a phase II trial of radical CCRT for T4/M1 LYM cancer that could not be surgically treated (21). The indications of this therapy have since expanded to unresectable cases. Thus, we did not perform chemotherapy with best supportive care alone but instead added CCRT if the patient’s condition was good.
However, the prognosis of Stage IVB esophageal cancer remains poor. Previous studies conducted in Japan for advanced esophageal cancer have reported a 3-year survival rate of approximately 20% (21), a 1-year survival rate of approximately 20% (22), and a MST of 305.5 days (23). In our study, the MST was 5 months, which is poor compared with those of previous studies using palliative therapies, including CCRT. One reason might be that the clinical stage was defined radiologically in our study. Patients who had lymph node metastases that become clear after surgery by microscopic examination and preoperatively unsuspected nodal disease may achieve good outcomes (24). Another possible reason is that the clinical stages of our subjects may have been more aggressive than those in previous studies. Twenty-one patients had organ metastases (including two sites), and all patients had N1 disease. Eleven patients had N3 disease according to the UICC 7th edition.
There have also been a few studies regarding predictors of OS. Several studies have also reported on the radiation doses used for stage IV esophageal cancer. Unresectable advanced esophageal cancer is currently treated by using concurrent CCRT consisting of RT at a dose of 60 Gy/fractions and FP therapy (25). However, Jingu et al. compared the results for patients irradiated by using >60 Gy with the results for patients irradiated by using ≤60 Gy but found no significant differences in OS or toxicities (14). Ikeda et al. studied the use of chemotherapy and palliative 40 Gy for stage IV esophageal cancer, and showed a MST of 308 days (10.3 months), and the 1-year-survival rate was 45.0% at a comparable to 50–60 Gy (15). In the present study, there were no significant differences in OS between ≤50 and >50 Gy. The worldwide standard of total 50.4 Gy irradiation for esophageal cancer without distant metastasis was used, and treatment was given with palliative intent, so the radiation dose of 50 Gy was thought to be reasonably safe. Previous studies have investigated age, distant metastasis, baseline white blood cell count, change in standardized uptake value in PET after chemotherapy, lack of anorexia/cachexia, and lack of widely disseminated disease as predictors of OS (26,27). We found that a small group of eight patients who underwent surgery after CCRT had more favorable OS than that of patients who did not have surgery. A few small studies have suggested that favorable OS can be achieved after surgery of metastatic esophageal cancer (28). Ohtsu et al. reported that the results for patients with M1 LYM were better than those for patients with T4 but without M1 LYM (21). Therefore, there is a possibility that aggressive control of the primary region is more effective if non-regional metastases are small and not widespread. It is thought that the patients who show PR and SD after CCRT benefit the most from surgery because the primary site residual tissue is removed. Even if there are non-regional small metastases, surgery may be effective for some patients. However, it is thought that the patient’s background influences the results. Patients who receive surgery tend to have less widespread metastasis than that of patients who only receive CCRT. Surgery can typically only be performed if a patient’s general condition is relatively good. Patients who have widespread organ metastases or T4 disease are excluded from surgery. For this select subgroup, surgery may still be beneficial and provide longer-term survival in some patients. However, the small number of patients who received surgery in the present study may have selection bias and short MST (11 months), so the medical care costs and burden on the patient of surgery should be carefully considered.
The objective CR rates of the primary lesion following a definitive CCRT dose of 60 Gy have been reported to be 62% in T3 cases and 37% in T4 cases (29). Ohtsu et al. reported a 42% clinical CR after high-dose CRT in patients with M1 (node). Use of high doses can often be expected to provide radical cures (21). On the other hand, Ikeda et al. reported the delivery of 40 Gy in a 20-Fr in combination with CCRT consisting of CDDP plus 5-FU, and the overall response rate, including for patients with metastatic lesions, was 55% (15). In our small subset of patients who received 40 Gy and surgery, the CR rate for the primary lesion was 37.5% (3/8) according to pathological findings. However, the objective response may not change much at 40 Gy. CCRT with 40 Gy cannot provide better results than those for high-dose RT but may provide acceptable results for patients with distant metastases.
Previously, some studies have demonstrated improvement in the rate of dysphagia. The radiation dose of palliative intent for esophageal cancer with metastasis reportedly ranges from 30 to 50 Gy. Hayter et al. reported the use of 30 Gy delivered in a 10-Fr stent in combination with CCRT consisting of 5-FU and mitomycin C in 22 patients with advanced incurable esophageal cancer (30). Burmeister et al. reported the use of 30 to 35 Gy with 5-FU for advanced esophageal cancer (11). In these studies, the improvement rates of dysphagia were 68% and 67%, respectively. However, the type of anticancer agent used in those studies differed from that used in our study, but we achieved better results possibly because we used a higher dose. However, there were no significant differences in the symptom and objective responses between ≤50 and >50 Gy. More than 50 Gy may not be necessary for stage IV esophageal cancer. However, ≥40 Gy appears to be necessary to improve the rate of symptom response.
There have been some studies on the toxicity of CCRT for esophageal cancer. Grade 3 and 4 toxicities have been observed in approximately 20% of patients with advanced esophageal cancer treated with CCRT (8,9,15). For definitive CCRT with 50–70 Gy, toxicities have been observed in >20% of patients (11,21,25,31). In the present study, the incidence of Grade 3 was 50% (17 patients). Fourteen patients had Grade 3 leukocytopenia, but only three patients had Grade 3 side effects except for leukocytopenia. Grade 3 leukocytopenia was able to be treated with an injection of G-CSF. A higher dose could lead to better and longer dysphagia relief, but the toxicities may become severe. It is important to balance objective and symptom responses with the potential toxicities associated with higher doses, especially in patients who cannot expect a cure.
The retrospective nature of this study and small number of patients whose backgrounds were medically diverse within a single center are limitations, but they do not detract from the significance of these preliminary findings. The relatively short follow-up duration and small sample size are key limitations of our study, and the results of the multivariate analyses were unclear. Another weak point of this study was the lack of proof of non-regional lymph node metastasis before CCRT. PET was performed only in a small number of patients; therefore, identification of metastasis in some patients may not have been accurate because only CT examinations were performed. Many of the patients received CCRT with palliative intent. Some patients were then transferred to other hospitals immediately after CCRT, and others were lost to follow-up. The acute toxicity rate was accurately assessed in our study, although the late toxicity rate could not be assessed because of the short duration of follow-up. Only eight patients received surgery. Maybe selection bias of these patients could be associated with better prognosis. Although the types of surgical procedures for esophageal cancer in our study were basically right-side transthoracic esophagectomy and reconstructed esophagus post sternum, two patients received total gastrectomy, and lymph node dissection was not performed. Thus, the date of surgery did not reflect the results of standard operation. These limitations may be addressed in the future studies. In this study, the clinical outcomes of stage IV esophageal cancer were poor, which was consistent with the outcomes in many previous studies. CCRT with 50 Gy gave results comparable to those of 60 Gy, which has been reported previously, and the toxicity was acceptable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study analyzed data on diagnosis and treatment. All procedures performed were in accordance with the ethical standards of the institutional review board and national research committees and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patient records/information was anonymized and de-identified prior to analysis in this study. Written patient’s consent to participate can be waived by institutional review board because of retrospective study. We informed all patients at the start of treatment that their treatment data may be used in future research, even if the treatment outcome was death. All patients agreed and consented to the use of their data for future research. We informed merit and demerit of the chemoradiotherapy and operation and consent was obtained from all patients included in the study.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol 1999;26:12-20. [PubMed]
- Kato H, Tachimori Y, Watanabe H, et al. Evaluation of the new (1987) TNM classification for thoracic esophageal tumors. Int J Cancer 1993;53:220-3. [Crossref] [PubMed]
- The Japanese Society for Esophageal Diseases. Comprehensive Registry of Esophageal Cancer in Japan (1998, 1999) and Long-term Results of Esophagectomy in Japan (1988–1997). 3rd edn, 2002.
- Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187-97. [Crossref] [PubMed]
- Javle M, Ailawadhi S, Yang GY, et al. Palliation of malignant dysphagia in esophageal cancer: a literature-based review. J Support Oncol 2006;4:365-73. [PubMed]
- Hayashi K, Ando N, Watanabe H, et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol 2001;31:419-23. [Crossref] [PubMed]
- Bleiberg H, Conroy T, Paillot B, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer 1997;33:1216-20. [Crossref] [PubMed]
- Iizuka T, Kakegawa T, Ide H, et al. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol 1992;22:172-6. [PubMed]
- Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260-5. [Crossref] [PubMed]
- Burmeister BH, Walpole ET, Burmeister EA, et al. Feasibility of chemoradiation therapy with protracted infusion of 5-fluorouracil for esophageal cancer patients not suitable for cisplatin. Int J Clin Oncol 2005;10:256-61. [Crossref] [PubMed]
- Coia LR, Soffen EM, Schultheiss TE, et al. Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer 1993;71:281-6. [Crossref] [PubMed]
- Eloubeidi MA, Desmond R, Arguedas MR, et al. Prognostic factors for the survival of patients with esophageal carcinoma in the US: the importance of tumor length and lymph node status. Cancer 2002;95:1434-43. [Crossref] [PubMed]
- Jingu K, Umezawa R, Matsushita H, et al. Chemoradiotherapy for T4 and/or M1 lymph node esophageal cancer: experience since 2000 at a high-volume center in Japan. Int J Clin Oncol 2016;21:276-82. [Crossref] [PubMed]
- Ikeda E, Kojima T, Kaneko K, et al. Efficacy of Concurrent Chemoradiotherapy as a Palliative Treatment in Stage IVB Esophageal Cancer Patients with Dysphagia. Jpn J Clin Oncol 2011;41:964-72. [Crossref] [PubMed]
- World Health Organization: WHO Handbook for Reporting Results of Cancer Treatment. WHO Offset Publication No. 48. Geneva, Switzerland: World Health Organization, 1979.
- U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. May 29th, 2009. Available online: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf, accessed 18th May 2015.
- Ilson DH, Ajani J, Bhalla K, et al. Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol 1998;16:1826-34. [Crossref] [PubMed]
- Tanaka T, Fujita H, Matono S, et al. Outcomes of multimodality therapy for stage IVB esophageal cancer with distant organ metastasis (M1-Org). Dis Esophagus 2010;23:646-51. [Crossref] [PubMed]
- Lee GJ, Park MI, Gwoo S, et al. Comparison of treatments in patients with inoperable stage IV advanced esophageal cancer. Korean J Gastroenterol 2012;59:282-8. [Crossref] [PubMed]
- Ohtsu A, Boku N, Muro K, et al. Definitive chemoradiotherapy for T4 and/ or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol 1999;17:2915-21. [Crossref] [PubMed]
- Nishimura Y, Suzuki M, Nakamatsu K, et al. Prospective trial of concurrent chemoradiotherapy with protracted infusion of 5-fluorouracil and cisplatin for T4 esophageal with or without fistula. Int J Radiat Oncol Biol Phys 2002;53:134-9. [Crossref] [PubMed]
- Ishida K, Ando N, Yamamoto S, et al. Phase II Study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group Trial (JCOG9516). Jpn J Clin Oncol 2004;34:615-9. [Crossref] [PubMed]
- Shimada H, Shiratori T, Okazumi S, et al. Surgical outcome of patients with thoracic esophageal cancer positive for cervical lymph nodes. Hepatogastroenterology 2007;54:100-3. [PubMed]
- Iwase H, Shimada M, Nakamura M, et al. Concurrent chemoradiotherapy for locally advanced and metastatic esophageal cancer: long-term results of a phase II study of UFT/CDDP with radiotherapy. Int J Clin Oncol 2003;8:305-11. [Crossref] [PubMed]
- Wang J, Suri JS, Allen PK, et al. Factors Predictive of Improved Outcomes With Multimodality Local Therapy After Palliative Chemotherapy for Stage IV Esophageal Cancer. Am J Clin Oncol 2016;39:228-35. [Crossref] [PubMed]
- Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer 2006;14:999-1011. [Crossref] [PubMed]
- Chao YK, Wu YC, Liu YH, et al. Distant nodal metastases from intrathoracic esophageal squamous cell carcinoma: characteristics of long-term survivors after chemoradiotherapy. J Surg Oncol 2010;102:158-62. [Crossref] [PubMed]
- Tahara M, Ohtsu A, Hironaka S, et al. Clinical impact of criteria for complete response (CR) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol 2005;35:316-23. [Crossref] [PubMed]
- Hayter CR, Huff-Winters C, Paszat L, et al. A prospective trial of short-course radiotherapy plus chemotherapy for palliation of dysphagia from advanced esophageal cancer. Radiother Oncol 2000;56:329-33. [Crossref] [PubMed]
- Kaneko K, Ito H, Konishi K, et al. Definitive chemoradiotherapy for patients with malignant stricture due to T3 or T4 squamous cell carcinoma of the oesophagus. Br J Cancer 2003;88:18-24. [Crossref] [PubMed]