Management pathways for solitary pulmonary nodules
Introduction
This article is for the special issue on “Liquid Biopsy for Lung Cancer Early Detection” and is focused on the management of solitary nodules, especially in suspected or proven lung cancers.
Several guidelines and criteria for evaluation of pulmonary nodules were identified using PubMed and through manual searching of references in published literature; website homepages were also reviewed for this article.
Background
There are a number of disease states in which pulmonary nodules are detected; primary lung cancer; metastatic lung cancer; benign disease (e.g., infectious, noninfectious, congenital). Several guidelines suggest management pathways for pulmonary nodules that are suspicious for primary lung cancer. To distinguish primary lung cancer from metastatic lung cancer, the International Association for the Study of Lung Cancer (IASLC) Staging and Prognostic Factors Committee (SPFC) has proposed a strategy.
This article summarizes this proposal and several guidelines, and highlights the differences among them.
Several guidelines recommend that decisions should be made by a multidisciplinary team including a pneumologist, a radiologist, a pulmonary oncologist, a thoracic surgeon, and a pathologist. Every effort must be made to obtain all available information, i.e., previous radiological data, risk factors, disease history, family history, and compromised status. Unless the nodule is a confirmed malignancy, guidelines recommend follow-up by low-dose, thin section computed tomography (CT). Positron emission tomography (PET)-CT, non-surgical biopsy and/or surgical resection are required for further evaluation. Suitability for CT follow-up or further invasive evaluation also depends on life-expectancy and preference of patient.
Management of single solitary nodules
A solitary pulmonary nodule is defined as round, moderately well marginated, opaque and no longer than 3 cm in maximum diameter (1). Tables 1-6 summarize management guidelines.

Full table

Full table

Full table

Full table
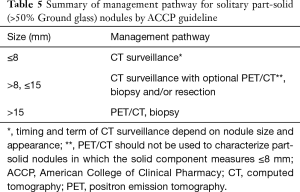
Full table

Full table
National Comprehensive Cancer Network (NCCN) guideline proposes different cutoffs for size, follow-up interval and surveillance term depending on the appearance of the nodule, e.g., solid, part-solid, or non-solid (2). Follow-up methods proposed by the Fleischner Society 2017 vary depending on whether the nodule is solid or sub-solid (part-solid or ground glass). The number of nodules, risk for lung cancer, and size or volume of each nodule is also considered for management strategy (3). American College of Clinical Pharmacy (ACCP) guidelines outline similar methodology; follow-up methods mainly depend on nodule appearance, nodule size, and risk or probability of malignancy (4,5). The interval or total term for follow-up and cutoff for nodule size vary between guidelines; however, guidelines agree that the initial evaluation should focus on appearance, i.e., solid, part-solid, or non-solid (ground glass). Sequentially, size and risk or probability of lung cancer are estimated. This methodology is also employed by lung CT Screening Reporting and Data System (lung-RADS) criteria (6), British Thoracic Society (BTS) guidelines (7), and the International Early Lung Cancer Action Project (I-ELCAP) (8).
Risk for lung cancer is evaluated by age, smoking history, symptoms, disease history (malignant neoplasm, chronic obstructive pulmonary disease, fibrosis etc.), and family history. Malignant probabilities are estimated in line with nodule characteristics such as size, margin, lobe location, internal characteristics (calcification, solid/non-solid component), growth speed, enhancement, and metabolism. If the nodule appears highly suspicious for malignancy, non-surgical biopsy or surgical resection should be carried out. Most guidelines recommend non-surgical biopsy or surgical resection when the nodule develops a solid component. The BTS suggests resection in cases where the nodule grows more than 2 mm in maximum diameter even if the nodule retains a pure ground glass appearance (9).
Management of multiple solitary nodules
The incidence of intrapulmonary metastasis (based on the TNM staging criteria, 7th edition) in the IASLC database is 2.94% (789/26859, T1a-4N[any]M[any] cases) (10). Based on the TNM 8th edition, the incidence in clinical and pathological N(any)M0 tumors (except contralateral pulmonary tumors) is 1.71% (505/29,595) and 3.56% (1,122/31,537), respectively (11). Another article reported that the frequency of cases with multiple nodules is up to 8% (12). The detection of lung cancer through low-dose CT screening (NELSON) clinical trial detected 1,222 new solid nodules in 11% of participants during the second or third screening round. Of them, 50 (4.09%) nodules were related to lung cancer (13).
Even if multiple solitary nodules are detected, the management method remains largely unchanged. The Fleischner Society recommends shorter follow-up intervals for patients with multiple nodules and intensive management based on the most suspicious nodule. NCCN and ACCP guidelines recommend management based on the largest nodules among multiple non-solid and solid nodules, respectively. Fundamentally, unless multiple nodules are suspected to be metastases, each nodule should be independently managed.
A couple of methodologies have been proposed regarding how to distinguish unrelated nodules from metastases (14-16). Histology, tumor location and existence of nodal/distant metastasis have been taken into consideration. The IASLC SPFC has recently proposed a new and detailed strategy summarized in Figure 1 (17,18). As strategies for single solitary nodule go, initial evaluation of single nodules is performed using radiology. Thereafter, histological characteristics are examined for further discrimination. Diagnosis will be proceeded with exclusion of possibility of metastasis by identifying the evidence which indicates the nodules are unlikely to be metastases. Nodules are diagnosed as metastases following the evaluation of several pathological features. Apart from internal appearance on CT and histological characteristics, metabolic uptake on PET-CT, ratio of growth, nodal or distant metastasis evaluation and different genetic features may help to distinguish unrelated tumors from metastases. The IASLC SPFC recommends handling nodules as unrelated in doubtful cases.
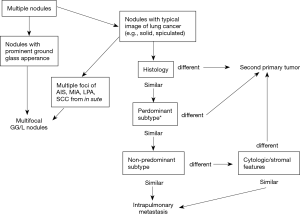
Importantly, different T descriptor must be used for staging of multifocal nodules, second primary tumors, and intrapulmonary metastasis (cases of intrapulmonary metastasis in the contralateral lung must be represented as M1a) (17).
Genomic profiling for multiple solitary nodules
Efforts have been made to discriminate multiple nodules on a genetic basis. A well-designed study focusing on epidermal growth factor receptor (EGFR) mutation (19) and another study using next-generation sequencing (20) indicated high homogeneous distribution of genetic alternations between primary and metastatic sites. Shared DNA rearrangements in multiple sites also favor metastases (21). Importantly, genetic profiling should be regarded as suggestive but not definitive (18). Although comprehensive analyses are warranted for definitive conclusion, they are not clinically available in most cases and unsophisticated analysis can lead to misleading conclusions. Unrelated tumors might harbor similar genomic profiles because they may arise due to shared risk factors (smoking exposure, damaged lung parenchyma, family history) in the same individual.
Liquid biopsy
Liquid biopsy is a non-invasive, highly sensitive and promising technology in early detection or therapeutic monitoring of lung cancer; several procedures and sample types can be utilized (22). However, diagnosis by liquid biopsy works on the premise that detected lung nodules harbor tumor-specific genetic alternations and additionally, those genetic alterations must stem from the lung nodule. There have been no studies validating liquid biopsy diagnosis for pulmonary nodules, especially early-stage lung cancer with ground glass appearance. Tissue biopsy is currently preferable for lung cancer diagnosis (23); further validation is required for liquid biopsy.
Discussion
This article has reviewed guidelines, IASLC SPFC proposal, and other literature regarding the management of solitary lung nodule(s). Each guideline has specific features and recommends different cutoff points, follow-up intervals or total surveillance terms and nodule appearance criteria. NCCN guidelines recommend measuring both whole nodule size and the size of the solid part in part-solid nodules. Further management algorithms for nodules during follow-up or PET-CT surveillance are also defined in detail. Fleischner guidelines recommend 3-dimensional CT (transverse, coronal, and sagittal) and nodule measurement based on the average long- and short-axis diameter, which can lead to useful volumetric measurement of nodules (with adequate software). There are several additional guidelines for the management of pulmonary nodules (6,7) and other clinical trials have looked at alternative management algorithms (8). In Asia, a modified version of ACCP guidelines has been advocated (24). Furthermore, there are models that can predict the likelihood of malignancy in pulmonary nodule based on CT and/or PET-CT appearance (25-27).
The NELSON study revealed that lung cancer probability in cases with nodules smaller than 5 mm or 100 mm3 is not significantly different from those without nodules (28). Although routine follow-up is not required for nodules with little probability of malignancy in some guidelines, there is discrepancy as to what characteristics indicate that follow-up is unnecessary. Different thresholds or criteria provide different false-positive ratios and sensitivity (29,30), and there are numerous variants in combination of guidelines and predictive models which define the risk of malignancy. However, there is no absolutely superior algorithm for management of pulmonary nodules. Accuracy or mortality might not be significantly different between guidelines as long as the algorithm is correctly utilized.
For multiple solitary nodules, there is a unique dilemma in deciding how to handle nodules that are either unrelated or metastases from a primary tumor. Management of multiple solitary nodules should be conducted according to guidelines and proposal. During surgical resection of suspicious nodules, aggressive exploration of the lymph nodes is warranted for accurate staging (31). Intrapulmonary metastatic cases are likely to be accompanied by lymph node metastasis and vice versa. In cases with lymph node metastasis, multiple solitary nodules are likely to be intrapulmonary metastasis (17). Metastasis in lymph node is no longer early-stage, needs further therapeutic strategy other than resection alone.
Although genetic examination does not provide a definitive diagnosis, it should be utilized in order to provide as much information as possible in addition to histology. If liquid biopsy is available, the genetic concordance between tissue and liquid biopsy might contribute further to the development of management algorithms. At present, diagnostic management by liquid biopsy seems difficult for multiple pulmonary nodules. It cannot be discriminated which nodule releases genomic alternation and diagnosis of intrapulmonary metastasis is challenging even with tissue comparison. Nevertheless, liquid biopsy remains promising in efforts to overcome unresolved issues. It has proven useful in the management of lung cancer and will hopefully be utilized more frequently, pending further validation.
Because there is no absolute standard for managing pulmonary nodule(s), the consensus should be based on multidisciplinary teams and the consent of patients, as well as their individual condition. Updated guidelines and proposals must be shared among multidisciplinary teams and the decision-making process should be documented. Management strategies based on precise records increase understanding and aid the development of publications, which will contribute to the progress of pulmonary nodule management. Genetic data collection will also be helpful for the progress and validation of liquid biopsy in the management of pulmonary nodule(s).
Conclusions
There are several guidelines and proposals for the management of solitary lung nodule(s). Management pathways vary but share the same principle; management decisions should be based on radiological appearance, size (volume), and risk for malignancy. Management of multiple solitary nodules is especially challenging. Liquid biopsy is promising but there are several problems that need to be solved prior to routine clinical use in pulmonary nodule management. The managing consensus should be based on multidisciplinary teams sharing guidelines and proposals.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for English language editing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Austin JH, Müller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327-31. [Crossref] [PubMed]
- Lung Cancer Screening Version 1. 2017. Available online: https://www.nccn.org/patients/guidelines/lung_screening/files/assets/common/downloads/files/lung_screening.pdf#search=%27nccn+guideline+lung+cancr+screening%27
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Lung-RADS™ Version 1.0 Assessment Categories. Available online: https://www.acr.org/Quality-Safety/Resources/LungRADS
- Baldwin DR, Callister ME. Guideline Development Group. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- International Early Lung Cancer Action Program. Available online: http://www.ielcap.org/
- Baldwin DR. Management of pulmonary nodules according to the 2015 British Thoracic Society guidelines. Key messages for clinical practice. Pol Arch Med Wewn 2016;126:262-74. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Detterbeck FC, Bolejack V, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Classification of Lung Cancer with Separate Tumor Nodules in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:681-92.
- Kim SS. The IASLC lung cancer staging project proposal for the classification of lung cancers with multiple pulmonary sites of involvement: the first step toward finding optimal treatment. J Thorac Dis 2016;8:2313-4. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Antakli T, Schaefer RF, Rutherford JE, et al. Second primary lung cancer. Ann Thorac Surg 1995;59:863-6; discussion 867. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Detterbeck FC, Nicholson AG, Franklin WA, et al. The IASLC Lung Cancer Staging Project: Summary of Proposals for Revisions of the Classification of Lung Cancers with Multiple Pulmonary Sites of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol 2016;11:639-50.
- Detterbeck FC, Franklin WA, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposed Criteria to Distinguish Separate Primary Lung Cancers from Metastatic Foci in Patients with Two Lung Tumors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:651-65.
- Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 2011;29:2972-7. [Crossref] [PubMed]
- Vignot S, Frampton GM, Soria JC, et al. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol 2013;31:2167-72. [Crossref] [PubMed]
- Murphy SJ, Aubry MC, Harris FR, et al. Identification of independent primary tumors and intrapulmonary metastases using DNA rearrangements in non-small-cell lung cancer. J Clin Oncol 2014;32:4050-8. [Crossref] [PubMed]
- Molina-Vila MA, Mayo-de-Las-Casas C, Giménez-Capitán A, et al. Liquid Biopsy in Non-Small Cell Lung Cancer. Front Med (Lausanne) 2016;3:69. [Crossref] [PubMed]
- Sholl LM, Aisner DL, Allen TC, et al. Liquid Biopsy in Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2016;140:825-9. [Crossref] [PubMed]
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation andadded value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490-6. [Crossref] [PubMed]
- Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretestprobability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383-8. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Gierada DS, Pinsky P, Nath H, et al. Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. J Natl Cancer Inst 2014.106. [PubMed]
- Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162:485-91. [Crossref] [PubMed]
- Rami-Porta R. Leave no lymph nodes behind! Eur J Cardiothorac Surg 2013;44:e64-5. [Crossref] [PubMed]


