Transvenous phrenic nerve stimulation, a novel therapeutic approach for central sleep apnea
Heart failure (HF) is a common disorder with a poor prognosis that can be improved by treating associated conditions such as sleep apnea. The combined prevalence of obstructive (OSA) and central (CSA) sleep apnea in HF patients has been estimated at 40–80% (1). CSA presents as apnea with a duration longer than 10 seconds without simultaneous thoracic and abdominal respiratory movement. Cheyne-Stokes respiration (CSR) is a special type of CSA characterized by a cyclic pattern of waxing and waning ventilation interrupted by episodes of central apnea or hypopnea that cause arousal, hypoxemia, reduced sleep quality, enhanced sympathetic nerve activation, pulmonary artery hypertension, and increased risk of sudden death (2).
Pathogenesis of CSA
There are at least three physiological causes of CSA. The first is increased central sensitivity to changes in blood PaCO2 that leads to hyperventilation followed by CSA induced by even small oscillations in partial pressure (3). This is regarded as the most important cause, as treatment by phrenic nerve stimulation (PNS) elevates end-tidal CO2 levels and improves CSA (4). The second is delayed circulation time. HF can prolong blood circulation time that results in a delay in detection of instantaneous changes of PaCO2 y chemoreceptors and the responses of effector organs such as the lungs. If the negative feedback response to hypocapnia becomes positive feedback because of delayed circulation time, then CSA is aggravated (5). The third cause is enhanced loop gain (LG). Increased of LG (LG >1) always associated with decreased of PaCO2, leading to the occurrence of CSA (6). PNS may influence LG, and it would be interesting to investigate the nature of the feedback loop, describe the changes in LG that occur during PNS, and determine whether LG can predict the suppressive effect of PNS on CSR in chronic HF (CHF) patients (7).
Current opinion is that CSR in CHF patients is associated with hypersensitivity to PaCO2 during sleep (8). The key pathophysiological cause of CSR is the oscillation of blood CO2 levels below and above the apneic threshold, and PaCO2 is normally maintained within a narrow range. Patients with CHF and CSA have a brisker ventilatory response to CO2 than those without CSA (9).
Traditional treatment of CSA
The current CSA treatments include continuous positive airway pressure (CPAP), adaptive servo ventilation (ASV), oxygen therapy, and CO2 inhalation. Each treatment is effective, but each has limitations. Compliance to CPAP treatment by HF patients is lower than that to other treatments (10). ASV was reported not to improve 6-month cardiovascular outcomes (11), and oxygen therapy was found to be only weakly effective (12). CO2 inhalation requires close monitoring during treatment, poses a risk of improper hypercapnia, and is difficult in home use (13). Novel and effective CSA treatments would be of great benefit.
Principle and mechanisms of PNS
PNS was first described by Sarnoff in 1951 (14), who reported that application of unilateral electric PNS resulted in reversible temporary inhibition of central respiratory control. However, the practical application and clinical significance of this phenomenon was not realized at that time. In spontaneously breathing animals, central inhibition of breathing is induced by selective activation of feedback from stretch receptors in the lung via the afferent vagal pathway.
An understanding of the anatomy of the phrenic nerve pathway is essential for performing PNS. After leaving the brain, the phrenic nerve passes through the neck and the thoracic cavity before finally reaching the diaphragm. Isolation of the phrenic nerve in the neck via open surgery is invasive and poses undue risk to surrounding vital nerves and disruption of the neck musculature. The phrenic nerve passes along the wall of the thoracic cavity adjacent to several veins, which allows transvenous electrode stimulation of the nerve. To stimulate phrenic nerve within thoracic cavity, a transvenous approach is not only anatomically feasible but also non- or minimally invasive.
The phrenic nerves pass close to the right wall of the superior vena cava or the right brachiocephalic vein in right thoracic cavity and along the wall of the left pericardiophrenic vein in left thoracic cavity. In several previous studies, the stimulation electrode was placed within the brachiocephalic or in left pericardiophrenic vein, as these sites are easily reached and fixed using percutaneous routes. In our previous study (7), PNS slowed the breathing rate during hyperpnea and increased PaCO2 by detecting the start of a hyperventilation episode, unilaterally stimulating the phrenic nerve to reduce the effect of spontaneous breathing and allowing PaCO2 to rise, and keeping CO2 above the apneic threshold, thus preventing apnea (Figure 1).
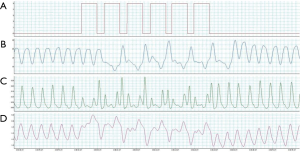
To position the leads connected to the phrenic nerve stimulator (Respicardia Inc., Minnetonka, MN, USA), venous access was obtained via the right subclavian vein. The lead used to provide PNS was positioned in the right brachiocephalic vein (7,8), and a sensing lead was placed in the azygos vein to record CSR. After successful performance of the transvenous PNS, an implantable pulse generator was placed in the right pectoral area (Figure 2). Selection of the optimal location of the stimulation electrode was guided by fluoroscopic visualization of diaphragm movement, palpation of the diaphragm, and patient feedback. After implantation, the amount of PNS was titrated monthly as needed to eliminate CSR events without arousing the patient from sleep (15). The intensity of PNS was modulated transdermally by an external magnetic guide device (Respicardia Inc., Minnetonka, MN, USA). PNS was automatically performed nightly following the patient's regular sleeping and waking times (Figure 3).
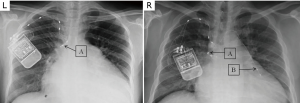
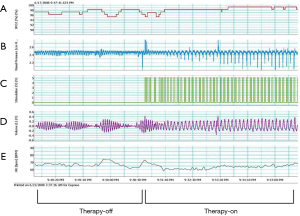
Studies of PNS with the remedē ® system for treating CSA
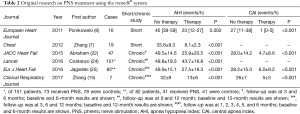
Fourteen studies describing the use of transvenous PNS have been published (Table 1); five are original research articles (Table 2). A total of 322 patients were included, 35 with temporary transvenous PNS without stimulator implantation and 287 patients with permanent transvenous PNS and stimulator implantation.

Full table
Full table
Ponikowski’s group and our team are the pioneers of this method, and were the first to report the successful clinical use of the remedē transvenous PNS stimulator or treatment of CSA/CSR in HF patients (7,8). The first PNS stimulator was implanted after completion of clinical trials of temporary PNS, and a total of seven HF patients experienced successful implantation with 6 months of follow-up. Remarkable efficacy was observed in these patients (15). A large multicenter study conducted in Europe and the USA found therapeutic efficacy, with elimination of CSA events and elevation of SpO2 compared with no PNS during long-term follow-up of 151 HF patients (24). A prospective randomized controlled study of 173 patients is underway. The most common adverse events reported included concomitant device interaction, implant site infection, swelling and local tissue damage, or pocket erosion. The remedē® system should not be used by patients with an active infection or by patients who are known to require magnetic resonance imaging.
On October 6, 2017, the FDA approved the use of the remedē® system to treat moderate to severe CSA (27). The device is not intended for use in patients with OSA, a condition in which the patient attempts to breathe, but the upper airway is partially or completely blocked. In conclusion, remedē® system and related PNS methods provide a novel treatment option for CSA patients.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (No. 81500069).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007;9:251-7. [Crossref] [PubMed]
- Brenner S, Angermann C, Jany B, et al. Sleep-Disordered Breathing and Heart Failure: A Dangerous Liaison. Trends in Cardiovascular Medicine 2008;18:240-7. [Crossref] [PubMed]
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999;341:949-54. [Crossref] [PubMed]
- Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol 2013;3:141-63. [PubMed]
- Hall MJ, Xie A, Rutherford R, et al. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med 1996;154:376-81. [Crossref] [PubMed]
- Sands SA, Edwards BA, Kee K, et al. Loop gain as a means to predict a positive airway pressure suppression of Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 2011;184:1067-75. [Crossref] [PubMed]
- Zhang XL, Ding N, Wang H, et al. Transvenous phrenic nerve stimulation in patients with Cheyne-Stokes respiration and congestive heart failure: a safety and proof-of-concept study. Chest 2012;142:927-34. [Crossref] [PubMed]
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012;33:889-94. [Crossref] [PubMed]
- Ponikowski P, Zhang SJ, Witkowski T, et al. Central Sleep Apnea Events Are Terminated by Phrenic Nerve Stimulation. J Am Coll Cardiol 2010;55:A30.E289.
- Kasai T, Narui K, Dohi T, et al. Efficacy of nasal bi-level positive airway pressure in congestive heart failure patients with cheyne-stokes respiration and central sleep apnea. Circ J 2005;69:913-21. [Crossref] [PubMed]
- O'Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular Outcomes With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure: The CAT-HF Trial. J Am Coll Cardiol 2017;69:1577-87. [Crossref] [PubMed]
- Sasayama S, Izumi T, Seino Y, et al. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J 2006;70:1-7. [Crossref] [PubMed]
- Andreas S, Weidel K, Hagenah G, et al. Treatment of Cheyne-Stokes respiration with nasal oxygen and carbon dioxide. Eur Respir J 1998;12:414-9. [Crossref] [PubMed]
- Sarnoff SJ, Sarnoff LC, Wittenberger JL. Electrophrenic respiration. VII. The motor point of the phrenic nerve in relation to external stimulation. Surg Gynecol Obstet 1951;93:190-6. [PubMed]
- Zhang X, Ding N, Ni B, et al. Safety and feasibility of chronic transvenous phrenic nerve stimulation for treatment of central sleep apnea in heart failure patients. Clin Respir J 2017;11:176-84. [Crossref] [PubMed]
- Cao M, Guilleminault C. Sleep-disordered breathing, heart failure, and phrenic nerve stimulation. Chest 2012;142:821-3. [Crossref] [PubMed]
- Augostini R. A novel approach to the treatment of central sleep apnea in patients with heart failure. Herzschrittmacherther Elektrophysiol 2012;23:9-13. [Crossref] [PubMed]
- Floras JS. Transvenous phrenic nerve stimulation for central sleep apnoea in heart failure: chicken or egg? Eur Heart J 2012;33:810-2. [Crossref] [PubMed]
- Oldenburg O, Bitter T, Fox H, et al. Effects of unilateral phrenic nerve stimulation on tidal volume. First case report of a patient responding to remede(R) treatment for nocturnal Cheyne-Stokes respiration. Herz 2014;39:84-6. [Crossref] [PubMed]
- Germany R, Joseph S, James K, et al. A novel therapeutic approach for the treatment of central sleep apnea: The remede(R) system. Cardiovasc Revasc Med 2014;15:235-9. [Crossref] [PubMed]
- Costanzo MR, Augostini R, Goldberg LR, et al. Design of the remede System Pivotal Trial: A Prospective, Randomized Study in the Use of Respiratory Rhythm Management to Treat Central Sleep Apnea. J Card Fail 2015;21:892-902. [Crossref] [PubMed]
- Abraham WT, Jagielski D, Oldenburg O, et al. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015;3:360-9. [Crossref] [PubMed]
- Naughton MT. Phrenic nerve stimulation for central sleep apnea: wiping out apnea or whipping the muscles? JACC Heart Fail 2015;3:370-2. [Crossref] [PubMed]
- Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016;388:974-82. [Crossref] [PubMed]
- Jagielski D, Ponikowski P, Augostini R, et al. Transvenous stimulation of the phrenic nerve for the treatment of central sleep apnoea: 12 months' experience with the remede((R)) System. Eur J Heart Fail 2016;18:1386-93. [Crossref] [PubMed]
- Joseph S, Costanzo MR. A novel therapeutic approach for central sleep apnea: Phrenic nerve stimulation by the remede(R) System. Int J Cardiol 2016;206 Suppl:S28-34. [Crossref] [PubMed]
- FDA News Release: FDA approves implantable device to treat moderate to severe central sleep apnea. 2017. Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm579506.htm


