Aortic arch repair under moderate hypothermic circulatory arrest with or without antegrade cerebral perfusion based on the extent of repair
Introduction
Since Griepp and colleagues (1) first introduced the concept of hypothermic circulatory arrest (HCA) for aortic arch surgery in 1970s, a number of surgical strategies involving various cerebral protection maneuvers have been developed for decades in efforts to improve the outcomes of arch surgery. With these advances, a series of studies have reported markedly improved outcomes of arch surgery (2-4). Despite these reports, however, recent analyses from large registries that may be more representative of real world results have shown that surgical risks of open arch surgery remain significantly high with operative mortality rate of 8.3–18.0% and neurologic injury rate of 6.6–8.3% (5-7). Among the important issues surrounding the arch surgery, achieving adequate cerebral protection is a primary concern, which involves complex cardiopulmonary bypass (CPB) strategies for the surgery.
Although profound or deep HCA has been traditionally regarded as a standard procedure for neuroprotection during arch repair, a recent meta-analysis revealed more favorable results of moderate HCA coupled with antegrade cerebral perfusion (ACP) over deep HCA (8). In addition, a recent large scale analysis from the German Registry for Acute Aortic Dissection Type A (GERAADA) (7) demonstrated that addition of ACP may be not necessary for aortic arch surgery when circulatory arrest time is less than 30 minutes. Even in cases where ACP is needed for more extended arch repair in that study, equivalent or even better neurological outcomes were demonstrated in patients undergoing unilateral ACP compared with those undergoing bilateral ACP.
Based on these notions (7,8), we endeavored to essentialize and simplify the procedures for aortic arch surgery from 2012. This involves distinct neuroprotective strategies according to the complexity of arch repair: hemi-arch replacement under moderate HCA alone and total-arch replacement under moderate HCA combined with unilateral ACP. This study aims to evaluate the safety and feasibility of these approaches for the conventional open arch repair.
Methods
From January 2012 through April 2017, 138 patients undergoing aortic arch repair due to either acute aortic syndrome or chronic arch aneurysm by a single surgeon (JBK) at Asan Medical Center, Seoul, Korea were enrolled in this study. A retrospective review of the patients was conducted. The study was approved by the institutional ethics committee/review board of the Asan Institute for Life Sciences (No. 2016-033), and the requirement for informed patient consent was waived in view of the retrospective nature of the study.
Surgical procedures and neurologic monitoring
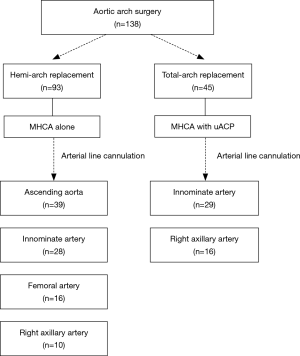
Distinct neuroprotective techniques were employed in hemi-arch and total-arch replacement (Figure 1). Indications of total-arch replacement in the setting of acute aortic syndrome were as follows: (I) primary entry tear cannot be excluded by hemi-arch replacement such as retro-type A aortic dissection or tear at the greater curvature, (II) complications related with arch involvement (malperfusion, rupture, or aneurysm >55 mm), and (III) in patients associated with connective tissue disorders such as Marfan syndrome.
We routinely applied bilateral cerebral oximeters using near-infrared spectroscopy for neuromonitoring in all patients. Initial baseline value of cerebral tissue oxygen saturation was obtained at pre-induction of anesthesia, and the trend is monitored based on the amount of change from an established baseline cerebral oxygenation value. Especially, unilateral significant decrease (>25% from the baseline) in left side cerebral oxygen saturation during unilateral ACP was regarded as an indication for intervention such as additional contralateral cerebral perfusion through left common carotid artery. Electroencephalography monitoring and other neuroprotective strategies such as corticosteroids or mannitol were not used. Every patient who presented with neurologic symptoms was assessed and evaluated by experienced neurologists, in which outpatient follow-up was also achieved by neurologist after discharge.
Hemi-arch replacement
In patients who were planned to undergo hemi-arch replacement, adjunctive ACP was not used since anticipated circulatory arrest time was less than 30 minutes. We targeted nasopharyngeal temperature of 25 °C. Without need for ACP, we prefer ascending aorta with the best priority for arterial-line cannulation site, which can be most quickly and easily approachable without a need for an additional skin incision. When ascending aorta was not available, arterial inflow site was selected by the priority order among innominate, femoral or right axillary artery (Figure 1). Presence of dissection or atheroma at target artery in preoperative CT was regarded as contraindication for cannulation. Innominate artery should be also precluded if its internal diameter was less than 10mm because the smaller diameter did not seem to accept direct cannulation technique. Innominate artery was directly cannulated by an 18-Fr DLP arterial cannula (Medtronic, Inc., Minneapolis, MN) after a single purse-string suture. Because the rubber band placed at 1cm from the cannula tip, we fixed the cannula securely with around tissue. The right axillary artery was also directly cannulated after 5 cm of skin incision just below the clavicle by semi-Seldinger technique using a 16 or 18 Fr RMI® cannulae (Edwards Lifesciences, Irvine, CA, USA) as was used in femoral artery rather than cannulating through the side graft. When directly cannulating at innominate or axillary arteries, secure ascertainment of adequate backflow from the target artery has been regarded of paramount importance before initiating the CPB.
In an attempt to maximize time efficiency, we routinely cross-clamped mid-ascending aorta after initiating the CPB so as to facilitate the proximal aorta processing while cooling the patients. After the infusion of cardioplegia solution, root repair or simple aortic valve replacement was performed until the patient’s temperature reaching the 25 °C. By reaching the target temperature, proximal aortic processing was suspended. Then CPB was turned off and HCA started. Distal anastomosis was then performed during HCA using a 1-branched woven Dacron graft. In the aim of minimizing the circulatory arrest time, we just approximate the native aorta and graft with a single layer of continuous running suture using either 4-0 or 3-0 polypropylene. Then, full CPB resumed as soon after distal anastomosis was completed, which was followed by multiple pledgeted reinforced sutures placed along full around the distal anastomosis site under full CPB support. At this moment, we started raising the body temperature to the normal level. When initial arterial inflow cannula was placed at the ascending aorta, perfusion was resumed using the side branch of the aortic graft whereas pre-existing arterial inflow route was maintained for the innominate, axillary and femoral arteries. Suspended aortic root or valve procedures was then resumed, and proximal aortic anastomosis was completed.
Total-arch replacement
In cases of total-arch replacement, HCA with unilateral ACP was used for cerebral protection as circulatory arrest time might be extended more than 30 minutes. During neuromonitoring using cerebral oximeters, a significant unilateral drop (>25% from baseline) in the left side cerebral tissue oxygen saturation was regarded as an indication for additional left side cerebral perfusion by placing ballooned perfusion catheter to the left common carotid artery. Arterial inflow site was chosen between innominate artery and right axillary artery to facilitate ACP. ACP flow rates were controlled to maintain the perfusion rate of 10–15 mL/kg/min, and perfusion pressure was kept in 50 to 80 mmHg. During lower-body ischemia, distal aortic anastomosis was made using a 4-banched woven Dacron graft. To reduce the lower body ischemic time, distal anastomosis was executed in the same manner as mentioned above in the hemi-arch replacement. After lower body perfusion restarted using the side arm of the graft, left common carotid arterial anastomosis was followed, by which the left side cerebral perfusion was resumed. As we begin to raise patient’s temperature, proximal aortic anastomosis was performed before the revascularization of innominate and left subclavian arteries in order to reduce cardiac ischemic time. After the release of aortic clamping, innominate artery was anastomosed under beating heart and left subclavian revascularization finally followed.
Results
Baseline profiles
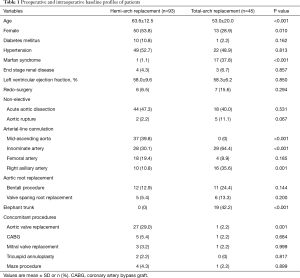
Of the 138 subject patients, 93 patients (67.4%) underwent hemi-arch replacement and 45 patients (32.6%) received total-arch replacement. The respective baseline intraoperative profiles are detailed in Table 1. Of 69 cases (50%) of non-elective cases, ATAAD and contained aortic rupture were presented in 62 patients (44.9%) and 7 patients (5.1%), respectively. Of the 62 dissection patients, root replacement procedure was performed in 14 patients (22.6%), 5 in hemi-arch replacement group and 9 in total-arch replacement group. Marfan syndrome was associated in 18 patients (13.0%), all of whom underwent total-arch replacement. Aortic root replacement either by Bentall procedure (n=9) or valve sparing procedure (n=6) was performed in all Marfan patients except three patients who underwent Bentall procedure in the previous operations.
Full table
Perioperative outcomes
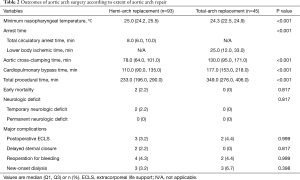
Overall, early-mortality occurred in 2 patients (1.4%) who underwent hemi-arch replacement due to acute type A aortic dissection (ATAAD) (Table 2). Both of these two patients underwent emergent hemi-arch replacement with conservative aortic root repair in the setting of ATAAD. The first patient was complicated by recurrent aortic root dissection associated with consequent aortic insufficiency and acute myocardial infarction at the postoperative 4th day. Although emergent operation consisting of redo-Bentall procedure and coronary artery bypass grafting was performed, the patient eventually died of low cardiac output syndrome. The second patient had underlying liver cirrhosis with a history of liver resection due to hepatocellular carcinoma. Even though main procedure was uneventful and TCA time took only 7 minutes in this particular patient, CPB weaning was unsuccessful because of intractable bleeding mainly from the aortic root attributable to coagulopathy. For acute aortic syndrome, early-mortality rate was 2.9% (2/69), and there were no mortality in the elective setting (0/69). New postoperative neurologic complications occurred in 2 patients (1.4%) in the hemi-arch repair group, all of which were temporary neurologic deficit. The first patient, who underwent hemi-arch repair in an elective setting due to atherosclerotic proximal arch aneurysm, experienced momentary neurologic deficit presented with loss of orientation and visual disturbance at the postoperative 7th day, but this symptom subsided within 10 minutes without any sequelae. Brain MR Image confirmed multiple micro embolic brain infarctions. The second patient presented with altered level of consciousness at the postoperative 2nd day after hemi-arch and valve sparing root replacement due to ATAAD. Bilateral internal carotid artery border zone infarction was observed in the brain MR examination, which may be attributable to postoperative temporary arch vessel hypoperfusion. The symptom, however, had been rapidly improved with supportive care and become fully restored at discharge at the postoperative 13th day without sequelae. Permanent neurologic dysfunction did not occur among the study population.
Full table
More specifically in the hemi-arch repair group (n=93), there were 2 cases (2.2%) in which the HCA time exceeded over 30 minutes, 33 and 41 minutes, respectively. Detailed time frames are described in Table 2. Among those 49 patients (52.7%) who received isolated hemi-arch repair including 38 cases (77.6%) of ATAAD, total procedure was completed in 3 hours in 26.5% (13/49), 4 hours in 67.3% (33/49) and 5 hours in 83.7% (41/49). Besides the two early mortalities and the two cases of temporary neurologic dysfunction, 8 patients required re-operation in the hemi-arch group. Four patients underwent re-exploration due to postoperative mediastinal bleeding. Three patients should have undergone re-operation because of postoperative aortic root complications, which include a mortality case aforementioned above. Among the rest two patients, one patient presented with root pseudoaneurysm showing contained rupture at the proximal anastomosis site in the immediate postoperative follow-up CT images, and therefore simple repair was performed. The other patient complicated with recurrent root dissection combined with aortic insufficiency underwent redo-Bentall procedure. At last, a patient, who presented with right ventricular dysfunction in the immediate postoperative echocardiography, showed extension of dissection in the right coronary artery with thrombosis in the CT images, and thus the patient should have undergone emergent off-pump CABG.
In the total-arch repair group (n=45), additional left side cerebral perfusion was indicated in 1 patient (2.2%). There were no early mortality and neurologic complications in this total-arch repair group while 5 patients should have undergone reoperation: re-exploration for postoperative bleeding in 2, mitral valve replacement due to infective endocarditis in 1, femorofemoral bypass due to lower limb ischemia in the setting of ATAAD in 1 and an emergent coronary bypassing due to postoperative coronary insufficiency in 1. Other complications are detailed in Table 2.
Discussion
To achieve adequate cerebral protection during aortic arch surgery, HCA has been regarded as a standard procedure since Griepp and colleagues (1) first reported their strategy, and profound or deep hypothermia have been used aiming to achieve the level of maximal suppression of cerebral metabolic rate; however, concerns over the adverse physiologic consequences of the profound hypothermia led to the introduction of milder degrees of hypothermia coupled with adjunctive cerebral perfusion (3). Although retrograde cerebral perfusion (RCP) has been widely used and is still regarded as a viable option for neuroprotection, the actual efficacy to oxygenate the brain has been questioned, and it has been shown that retrograde perfusion cannot provide adequate cerebral perfusion by an in vivo study (9). Meanwhile ACP has become the most widely used technique worldwide in recent years, for instance International Registry of Acute Aortic Dissection reported that ACP has been used in 66.1% of ATAAD surgeries from 2011 to 2016 (10). Although there have been debates over the methodology of ACP, recent large-scale studies have shown no significant differences between unilateral ACP group and bilateral ACP group with respect to the rates of death and neurologic deficits (7,11).
In the present study, we employed distinct cerebral protection strategies according to the extent of arch replacement. We performed hemi-arch replacement without ACP in a belief that HCA is enough when circulatory arrest time is less than 30 minutes based on the GERAADA trial, which revealed no differences between HCA alone and the addition of ACP when arrest time is less than 30 minutes and suggested 30 minutes as a cut-off point for use of ACP (7). This hypothesis was further supported by a nationwide analysis from Japan (12). In this cited study, a comparative analysis of HCA + ACP versus HCA alone in patients undergoing arch replacement was conducted, in which there were no significant differences in early mortality, incidence of stroke, and transient neurologic disorder. These recent observations served as hypothetical backgrounds for our hemi-arch replacement strategy of moderately cooled temperature without adjunctive cerebral perfusion (7,12).
We achieved considerably satisfactory early and neurologic outcomes with this approach. We have previously reported outcomes of hemi-arch and total-arch repair in the setting of acute type A aortic dissection (13). In the cited study, hemi-arch replacement and total-arch replacement was performed to treat acute type A dissection in 144 and 44 patients, respectively. Adjunctive cerebral perfusion such as ACP or RCP was employed in all cases, but 3 cases in hemi-arch repair. Early death occurred in 14 (9.7%) and 6 patients (13.6%) and permanent neurologic injury including stroke, paraplegia and coma occurred in 38 (26.4%) and 21 patients (47.7%), respectively. On the other hands, in the present study, there were only two early deaths (4.5%, 2/44) in hemi-arch group and no early death (0%, 0/18) in total-arch group in the management of acute type A dissection with this approach. Moreover, any permanent neurologic injury was not left. When performing crude actuarial comparison between the two study cohorts in the similar setting, there was a tendency in favor of the new approach in early mortality (3.2% vs. 10.6%, P=0.074) and neurologic outcomes were strongly superior in the new approach (0% vs. 31.4%, P<0.001).
Our belief regarding neuroprotection during open arch surgery is that minimization of arrest time is of crucial importance. As aforementioned above, real world result of neurologic injury rate is still unsatisfactory despite advent of various strategies of brain protection such as RCP or ACP. Anatomical variations of cerebral circulation system and individual condition of patient’s vessel like atherosclerotic burden complicate these procedures and result in unintended consequence of neurologic outcomes. In this context, we endeavored to minimize the arrest time by reducing the time consumption of distal aortic anastomosis for the purpose of un-necessitating adjunctive cerebral perfusion. We performed distal aortic anastomosis with a single layer continuous suture, which was reinforced by multiple interrupted pledgeted sutures under the restoration of distal perfusion. Brief arrest time permitted hemi-arch repair without additional perfusion so that we could perform hemi-arch repair “without-touching” arch vessels. However, it cannot be emphasized enough that the additive cerebral perfusion should be prepared on-ready whenever the arrest time happens to extend beyond the expected time. Indeed, we have been on-ready of additional cerebral perfusion even though there has been no such case during the study period for hemi-arch replacement.
Some concerns can be raised regarding safety issues around the single layer suture for distal anastomosis and its reproducibility of such brief arrest time. We believe, however, that this technique is more favorable for secure hemostasis and quality aortic reconstruction in that reinforce sutures can be placed under CPB without restriction of time and disturbance. In our view, the brief arrest time in hemi-arch repair is also fully reproducible. For instance, the study subjects include ten patients in whom the surgery was performed by trainee-level cardiac surgeons (residents, fellows)—circulatory arrest times for hemi-arch repair were less than 10 minutes in all of these cases without developing complications.
We preferred direct cannulation on the ascending aorta or innominate artery over right axillary cannulation for hemi-arch repair, but when needed, axillary artery was also directly cannulated using semi-Seldinger technique rather than using a side graft anastomosis. The safety and efficacy of central aortic cannulation have been well established by other previous reports (14,15). Innominate artery has been relatively less preferred site of direct arterial cannulation and limited data have been reported on its uses (16,17). Innominate artery cannulation, however, has both advantage of the central cannulation, which promptly facilitates the ACP without additional skin incision and minimizes risks of cerebral embolism attributable to retrograde aortic flow. In contrast to relatively time-consuming axillary artery cannulation, this technique can be also employed quickly in emergent cases. We concede that there are some concerns regarding local complications including dissection, tear, inadequate flow, or abutment of the cannula tip at direct innominate cannulation site. Thus strict patient selection should be preceded, and flexible management according to the flow and pressure status is essential. Although similar concerns also exist in direct axillary cannulation, risk of dissection and tear is relatively spared by using the semi-Seldinger technique. Additive femoral artery cannulation was implemented in four cases, however, because of inadequate inflow and pressure overloading to innominate or axillary cannulation. With this meticulous attention, local complications did not occur with the use of direct cannulation of 57 cases of innominate artery and 26 cases of axillary artery in our series.
Limitations
This study has inherent limitations of retrospective observational studies. The study reported outcomes of aortic arch surgery performed by a single surgeon in a high-volume center, and the results may not be therefore generalizable to other settings.
Although major permanent neurologic event was not found, long-term effect of our neuroprotective strategy on minor cognitive function is still unknown. Therefore, further validations on the neurologic outcomes using more sophisticated monitoring tool with larger sample size coupled with comparison group may be needed to confirm the findings of this study.
Conclusions
Despite remarkable technical development, aortic arch surgery is still a challenging procedure. We performed aortic arch surgery in a customized fashion by individual patient’s condition in two big stems of cerebral protection strategy; moderate HCA for hemi-arch replacement and HCA with unilateral ACP for total-arch replacement. The combination of several adjunctive procedures including initial single layer suture of distal anastomosis, simplified arterial-line cannulation and milder degree of hypothermia contributed to reduction in procedural time, and thereby surgeon was allowed to focus more on the main procedure. In consequence, we could achieve favorable overall outcomes with this approach with satisfactory safety as well as reasonable efficiency.
Acknowledgements
Funding: This study was supported by the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea [2016-033].
Footnote
Conflicts of Interest: Presented at the 25th Annual Meeting of the Asian Society for Cardiovascular and Thoracic Surgery, Seoul, Korea, March 23–26, 2017.
Ethical Statement: The study was approved by the institutional ethics committee/review board of the Asan Institute for Life Sciences (No. 2016-033), and the requirement for informed patient consent was waived in view of the retrospective nature of the study.
References
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63. [PubMed]
- Keenan JE, Wang H, Gulack BC, et al. Does moderate hypothermia really carry less bleeding risk than deep hypothermia for circulatory arrest? A propensity-matched comparison in hemiarch replacement. J Thorac Cardiovasc Surg 2016;152:1559-69.e2. [Crossref] [PubMed]
- Englum BR, Andersen ND, Husain AM, et al. Degree of hypothermia in aortic arch surgery - optimal temperature for cerebral and spinal protection: deep hypothermia remains the gold standard in the absence of randomized data. Ann Cardiothorac Surg 2013;2:184-93. [PubMed]
- Urbanski PP, Lenos A, Bougioukakis P, et al. Mild-to-moderate hypothermia in aortic arch surgery using circulatory arrest: a change of paradigm? Eur J Cardiothorac Surg 2012;41:185-91. [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol 2012;60:1156-62. [Crossref] [PubMed]
- Kruger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011;124:434-43. [Crossref] [PubMed]
- Tian DH, Wan B, Bannon PG, et al. A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann Cardiothorac Surg 2013;2:148-58. [PubMed]
- Ehrlich MP, Hagl C, McCullough JN, et al. Retrograde cerebral perfusion provides negligible flow through brain capillaries in the pig. J Thorac Cardiovasc Surg 2001;122:331-8. [Crossref] [PubMed]
- Parikh N, Trimarchi S, Gleason TG, et al. Changes in operative strategy for patients enrolled in the International Registry of Acute Aortic Dissection interventional cohort program. J Thorac Cardiovasc Surg 2017;153:S74-S79. [Crossref] [PubMed]
- Angeloni E, Benedetto U, Takkenberg JJ, et al. Unilateral versus bilateral antegrade cerebral protection during circulatory arrest in aortic surgery: a meta-analysis of 5100 patients. J Thorac Cardiovasc Surg 2014;147:60-7. [Crossref] [PubMed]
- Okita Y, Miyata H, Motomura N, et al. A study of brain protection during total arch replacement comparing antegrade cerebral perfusion versus hypothermic circulatory arrest, with or without retrograde cerebral perfusion: analysis based on the Japan Adult Cardiovascular Surgery Database. J Thorac Cardiovasc Surg 2015;149:S65-73. [Crossref] [PubMed]
- Kim JB, Chung CH, Moon DH, et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2011;40:881-7. [PubMed]
- Frederick JR, Yang E, Trubelja A, et al. Ascending aortic cannulation in acute type a dissection repair. Ann Thorac Surg 2013;95:1808-11. [Crossref] [PubMed]
- Kamiya H, Kallenbach K, Halmer D, et al. Comparison of ascending aorta versus femoral artery cannulation for acute aortic dissection type A. Circulation 2009;120:S282-6. [Crossref] [PubMed]
- Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg 2004;78:1274-84; discussion 1284. [Crossref] [PubMed]
- Banbury MK, Cosgrove DM 3rd. Arterial cannulation of the innominate artery. Ann Thorac Surg 2000;69:957. [Crossref] [PubMed]


