Long-term survival without surgery in NSCLC patients with synchronous brain oligometastasis: systemic chemotherapy revisited
Introduction
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related deaths worldwide. Among patients with NSCLC, 30–50% of patients have distant metastases and 7.4–10.0% have brain metastases at the time of diagnosis (1,2). Advanced stage NSCLC patients have a poor prognosis. The 5-year survival rate for these patients has been reported to be less than 10% (3). Among patients with metastatic NSCLC, brain metastases are correlated with a shorter survival time (4).
Hellman et al. proposed a clinically significant status known as oligometastasis, which was initially described as a restricted locoregional status (5). Oligometastasis is now-defined as a small number of isolated distant metastases (generally less than 5 sites) (6). An incidence rate of 4.0% has been reported for brain oligometastasis (BOM) as the sole distant lesion in patients with NSCLC (any N grade) receiving surgical treatment (7). A few studies have reported patients with TNM stage N0 to N1 BOM (N0–1 BOM) who did not receive surgical treatment.
The standard treatment for stage IV NSCLC is chemotherapy. A few studies have reported that patients with BOM who receive surgical treatments for both their primary and metastatic sites have a better prognosis (8-15). However, a comparison of the survival outcomes for NSCLC patients with BOM undergoing either surgery or chemotherapy for the primary site has not yet been performed.
The purpose of this retrospective study was to investigate the frequency and treatment outcome of patients with N0–1 BOM who received chemotherapy as a treatment for the primary site and definitive treatment for brain metastases.
Methods
Patients with stage IV lung cancer who received chemotherapy at the National Cancer Center Hospital in Japan between 2000 and 2010 were included in this analysis. We selected NSCLC patients with brain involvement (less than 5 metastases) as the sole distant lesion at the time of diagnosis (synchronous metastases) (6,16). Among those patients, N0–1 stage NSCLC cases who were a candidate for resection surgery of the primary lesion if they did not have brain metastases were included in this study. All the patients were evaluated using computed tomography (CT) examinations of the chest and abdomen, magnetic resonance imaging (MRI) and/or CT of the brain, and a bone scan and/or PET-CT if available as a standard staging work-up to investigate distant metastases before receiving chemotherapy. Patients were staged using the 7th edition of the Union for International Cancer Control TNM classification for lung cancer. Patient age, sex, performance status (PS), histology, information on driver oncogenes [EGFR, and anaplastic lymphoma kinase (ALK) rearrangement], TNM staging, number of brain metastases, maximum size of brain metastases, initial systemic treatment (chemotherapy or molecular targeted therapy), initial treatment for the brain metastases (surgery, whole brain irradiation, stereotactic radiotherapy), pattern of recurrence after initial treatment, and second-line systemic therapy regimens were collected. This study was approved by the institutional review board of the National Cancer Center Hospital [2014-160].
Statistical analysis
OS was defined as the interval between the date of initial treatment (systemic chemotherapy or local therapy for brain metastases) and the date of death or the most recent date of follow-up for surviving patients. PFS was measured from the date of initial treatment until death before progression, the date of disease progression, or the most recent date of follow-up for surviving patients without progression. PFS and OS were estimated using the Kaplan-Meier method. SPSS version 19 (SPSS, Chicago, IL, USA) was used for the statistical analyses.
Results
Patient characteristics
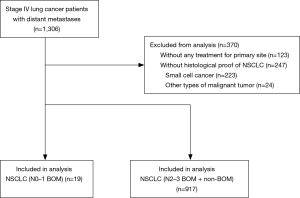
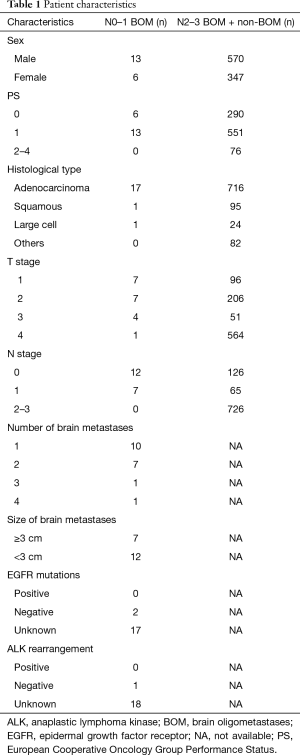
A total of 1,306 patients with stage IV NSCLC who were treated at our hospital were identified. The cohort diagram is shown in Figure 1. We excluded 370 patients: 123 patients who did not receive any treatment for the primary site, and 247 patients who did not have histological confirmation of NSCLC. We identified 19 patients (2.0%) with brain involvement (less than 5 metastases) as the sole distant lesion and N0 or N1 disease as N0–1 BOM patients and 917 stage IV NSCLC patients who received chemotherapy with or without radiation for treatment of the primary site as N2–3 BOM + non-BOM (no brain metastasis or more than 5 brain metastases) patients. The median duration of the follow-up period for the 19 N0–1 BOM patients was 16.0 months (range, 3.0 to 88.7 months). Four of the 19 patients were lost to follow-up but were not reported to have died, and one patient was still alive at the time of the study cut off (October 2016). The patient characteristics are summarized in Table 1. Among the N0–1 BOM patients, the histological findings showed 17 adenocarcinomas (89%), 1 squamous cell carcinoma (5%), and 1 large cell carcinoma (5%). One patient (5%) had an activated mutation (L858R) of the epidermal growth factor receptor (EGFR) gene, 3 patients (16%) had wild-type EGFR, and 15 patients (79%) had an unknown EGFR status. None of the patients had an ALK oncogene rearrangement, 1 patient (5%) had a negative result, and 18 patients (95%) were not examined. The brain metastases consisted of 1 site in 10 patients (53%), 2 sites in 7 patients (37%), 3 sites in 1 patient (5%), and 4 sites in 1 patient (5%). In 7 cases (37%), the maximum diameters of the brain metastases were more than 30 mm. Three patients (16%) received brain surgery, 5 patients (26%) received whole brain radiation therapy (WBRT), 5 patients (26%) received stereotactic radio surgery (SRS), and 6 patients (32%) received only systemic chemotherapy for the initial management of the brain metastases. The primary systemic therapies were cytotoxic chemotherapy in 17 patients (90%) and EGFR tyrosine kinase inhibitor (EGFR-TKI) in 2 patients (11%). All the first-line systemic cytotoxic chemotherapy regimens were platinum-based regimens.
Full table
Efficacy of chemotherapy and survival benefit
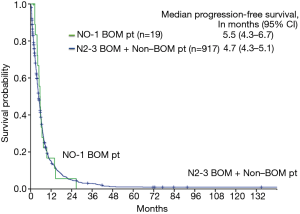
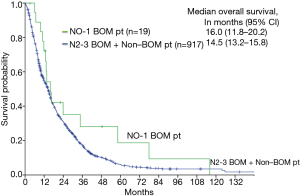
The median PFS of the N0–1 BOM patients was 5.5 months (95% CI, 4.3–6.7 months), while that of the N2–3 BOM + non-BOM patients was 4.7 months (95% CI, 4.3–5.1 months). The Kaplan-Meier curve for PFS is shown in Figure 2. Among the N0–1 BOM patients, 5 patients had local recurrences, 13 patients had distant metastases or mixed recurrences (3 with brain and thoracic sites, 1 with brain and distant sites, 1 with thoracic and distant sites, and 8 with distant metastasis). Among the 9 patients who developed brain recurrences, 6 patients had received brain radiotherapy (3 had received WBRT, and 3 had received SRS) and the other 3 patients had not received brain radiotherapy. Four patients (21%) had received EGFR-TKI and 8 patients (42%) had received cytotoxic agents. The median 3- and 5-year survival rates of the N0–1 BOM patients were 28% and 19%, respectively. The median OS of the N0–1 BOM patients was 16.0 months (95% CI, 11.8–20.2 months), while that of the N2–3 BOM + non-BOM patients was 14.5 months (95% CI, 13.2–15.8 months). There were no statistically difference between two arms in the OS (P=0.13) and PFS (P=0.782) using log rank test. The Kaplan-Meier curve for OS is shown in Figure 3. There were 2 long-term survivors with N0–1 BOM who survived for more than 5 years. One patient was followed without treatment for more than 5 years after one cycle of platinum-containing chemotherapy and palliative local radiation therapy. This patient died of disease progression. Another patient was observed without treatment for more than 2 years after receiving 6 lines of chemotherapy and radiation therapy for the brain metastases. Despite not having any of the common EGFR mutations, this patient received EGFR-TKI and survived without disease progression for 2 years.
Discussion
The frequency of N0–1 BOM was 2.0% among patients with stage IV NSCLC. The 3- and 5-year survival rates in patients with N0–1 BOM were 28% and 19%, respectively. This is the first report describing the treatment outcome of chemotherapy without surgical resection and radiotherapy for the primary site in NSCLC patients with brain involvement as a single-organ metastasis.
Few reports have described the incidence of oligometastasis and BOM among NSCLC patients without surgery for the primary site. Brain metastases were reportedly detected in 7.4–10% of patients with NSCLC at presentation and in 30–50% of patients during therapy (2,17). In a previous report, 5.5% of NSCLC patients had oligometastases (1). Among the NSCLC patients referred to surgical departments, 4.0% of NSCLC patients had BOM (7). We reported that the frequency of patients with N0–1 BOM was 1.8% among those patients who were treated without surgical resection. According to previous studies, the incidence of BOM might range from 1.8% to 5.5%.
Advanced NSCLC patients usually have a poor prognosis and rarely survive for as long as 5 years. The median survival period for advanced NSCLC patients with brain metastases was 7–9 months according to previous studies (4,18-20). Radiation therapy or surgical treatment for brain metastases improved the survival periods of these patients (21,22). The results of several randomized controlled trials have suggested three standard treatments for NSCLC patients with brain metastases: resection with sequential stereotactic radiation or whole brain irradiation, stereotactic radiation therapy alone, and stereotactic radiation therapy with sequential whole brain irradiation (18-20). Several reports have proposed that surgical treatment for the primary site improves the survival outcome, compared with chemotherapy +/− radiotherapy, but few reports have described the outcomes of cases treated with only chemotherapy +/− radiotherapy for the primary site. The leading cause of death among patients who have received local treatments for brain metastasis is the progression of primary and other metastatic lesions (23). There have been numerous published reports describing the combined resection of a primary lung site and a solitary brain metastasis (9,24,25). Surgical resection for both the brain and primary sites was suggested to prolong survival among NSCLC patients with a sole brain metastasis as early as 1976 (6,26). A recurrence-free rate of 6.3–14.0% at 4 years, a survival rate of 6.6–21.0% at 5 years, and a median survival time of 8.5–24.0 months were observed in trials including patients who underwent the resection of both the primary lesion and brain metastases (8,10,12,27). In trials using chemoradiotherapy for the treatment of the primary lesion and radiotherapy +/− resection for the brain metastases, the 2-year recurrence-free rate, the 5-year overall survival (OS) rate, and the median survival time were 53.0%, 7.6% and 13.1–31.8%, respectively (24,28,29). In the present study, the 5-year survival rate was 19% and the median survival was 16.0 months among the N0–1 BOM patients treated with chemotherapy for the primary site without surgical resection. This result suggests that the prognosis of patients without surgery was not worse than that of patients who received surgery for the primary site.
Patients with N0–1 BOM have a better prognosis, although the development of chemotherapy in recent years has contributed to an improvement in the survival outcome. The median OS of stage IV NSCLC patients with N0–1 BOM was 16 months, and this outcome was better than the OS of 6–9 months reported in previous trials among NSCLC patients who had brain involvement with or without other organ metastasis. According to the results of phase 3 trials among patients with advanced-stage unresectable NSCLC who received platinum-containing regimens (CDDP + PEM/CDDP + GEM/CBDCA + PTX), the median survival period was 10–18 months after initial chemotherapy (30-32). Although the survival time in our study was estimated after the treatment for the primary site or brain metastasis had begun, these results show that patients with N0–1 BOM have a relatively favorable prognosis (4,18-20). The survival outcome is expected to be further improved because of recent improvements in chemotherapies. Since one long-term survivor in this study received gefitinib for more than 2 years, the development of molecular target drugs is likely to improve the outcomes of NSCLC patients with N0–1 BOM.
Compared with previous reports, our results suggest that the treatment outcome of the N0–1 BOM patients without surgery for the primary site might be better than that of usual stage IV NSCLC patients and similar to that of those with chemoradiotherapy or surgery for the primary site. The guidelines established by the NCCN, ACCP, and ESMO include surgery for the primary lesion as a treatment option for patients with oligometastatic NSCLC and an N0–1 status. Several clinical trials evaluating the efficacy of chemoradiation therapy for NSCLC patients with oligometastases of distant lesions have been conducted (May, 2017). Further discussion of the role of surgery is needed based on balanced evidence comparing chemotherapy and surgical resection in oligometastatic NSCLC.
This report describes a relatively small retrospective study. Therefore, a selection bias might exist. Most of the patients in this study had been treated before the development of EGFR-TKIs, and the presence of EGFR mutations was not examined in these patients. In the present study, however, the impact of this circumstance on survival might be relatively small, since the number of patients treated with EGFR-TKIs after testing for EGFR mutations was also small. In oligometastatic brain metastases arm, three patients lost to follow up. The impact of these patients of survival analysis was small because all of them were considered that they were not capable of receiving additional chemotherapy, including EGFR-TKIs, for poor general condition. To further consider the development of molecular targeted drugs and immune-based therapy, further evaluation of driver mutations and the expression of programmed cell death-ligand 1 (PD-L1) is needed to select patients for future clinical trials. Addition to that, multi-institution study should be conducted, considering that it needs to find 19 patients for 10 years in single institution.
Conclusions
The frequency of N0–1 BOM patients with NSCLC who received chemotherapy for their primary sites was 2.0% (19 out of 936). The PFS among the NSCLC patients with N0–1 BOM who received chemotherapy without resection surgery for the primary site was similar to that of patients who received resection surgery. A randomized trial comparing the efficacy of chemotherapy for the primary site with or without surgery is warranted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of the National Cancer Center Hospital [2014-160]. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. [Crossref] [PubMed]
- Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 2004;45 Suppl 2:S253-7. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Oh Y, Taylor S, Bekele BN, et al. Number of metastatic sites is a strong predictor of survival in patients with nonsmall cell lung cancer with or without brain metastases. Cancer 2009;115:2930-8. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]
- Quint LE, Tummala S, Brisson LJ, et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg 1996;62:246-50. [Crossref] [PubMed]
- Magilligan DJ Jr, Duvernoy C, Malik G, et al. Surgical approach to lung cancer with solitary cerebral metastasis: twenty-five years' experience. Ann Thorac Surg 1986;42:360-4. [Crossref] [PubMed]
- Wroński M, Arbit E, Burt M, et al. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg 1995;83:605-16. [Crossref] [PubMed]
- Mussi A, Pistolesi M, Lucchi M, et al. Resection of single brain metastasis in non-small-cell lung cancer: prognostic factors. J Thorac Cardiovasc Surg 1996;112:146-53. [Crossref] [PubMed]
- Endo C, Hasumi T, Matsumura Y, et al. A prospective study of surgical procedures for patients with oligometastatic non-small cell lung cancer. Ann Thorac Surg 2014;98:258-64. [Crossref] [PubMed]
- Billing PS, Miller DL, Allen MS, et al. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 2001;122:548-53. [Crossref] [PubMed]
- Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest 2001;119:1469-75. [Crossref] [PubMed]
- Lo CK, Yu CH, Ma CC, et al. Surgical management of primary non-small-cell carcinoma of lung with synchronous solitary brain metastasis: local experience. Hong Kong Med J 2010;16:186-91. [PubMed]
- Saitoh Y, Fujisawa T, Shiba M, et al. Prognostic factors in surgical treatment of solitary brain metastasis after resection of non-small-cell lung cancer. Lung Cancer 1999;24:99-106. [Crossref] [PubMed]
- Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013;82:197-203. [Crossref] [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Weissman DE. Glucocorticoid treatment for brain metastases and epidural spinal cord compression: a review. J Clin Oncol 1988;6:543-51. [Crossref] [PubMed]
- Diener-West M, Dobbins TW, Phillips TL, et al. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys 1989;16:669-73. [Crossref] [PubMed]
- Chidel MA, Suh JH, Greskovich JF, et al. Treatment outcome for patients with primary nonsmall-cell lung cancer and synchronous brain metastasis. Radiat Oncol Investig 1999;7:313-9. [Crossref] [PubMed]
- Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Salvati M, Cervoni L, Tarantino R, et al. Solitary cerebral metastasis as first symptom of lung cancer. Neurochirurgie 1994;40:256-8. [PubMed]
- Magilligan DJ Jr, Rogers JS, Knighton RS, et al. Pulmonary neoplasm with solitary cerebral metastasis. Results of combined excision. J Thorac Cardiovasc Surg 1976;72:690-8. [PubMed]
- Getman V, Devyatko E, Dunkler D, et al. Prognosis of patients with non-small cell lung cancer with isolated brain metastases undergoing combined surgical treatment. Eur J Cardiothorac Surg 2004;25:1107-13. [Crossref] [PubMed]
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Regine WF, et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys 2008;72:19-23. [Crossref] [PubMed]
- Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763-74. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Paz-Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 2015;16:328-37. [Crossref] [PubMed]