Robot-assisted thymectomy via subxiphoid approach: technical details and early outcomes
Introduction
With benefits over trans-sternal thymectomy including less trauma and fewer surgical complications, such as myasthenic crisis, video-assisted thoracoscopic thymectomy through various approaches becomes increasingly applied in the surgical treatment of anterior mediastinal masses and myasthenia gravis (1). The trans-subxiphoid thoracoscopic thymectomy is a promising technique which was documented that can provide an excellent visualization of the upper pole of the thymus and the bilateral phrenic nerves as median sternotomy (2), so it becomes possible for adequate bilateral mediastinal fatty tissue dissection without sternotomy or cervical incision. However, the trans-subxiphoid thoracoscopic thymectomy is a technically demanding surgical procedure associated with ergonomic discomfort. Suda et al. (2) sought to improve its manoeuvrability with the use of the Da Vinci multi-joint robotic system. According to the description of Suda’ paper, a conventional thoracoscopy must be used to open bilateral pleura before the robotic instruments reach the operative field in the anterior mediastinum. Herein, we introduce a modified procedure of robot-assisted trans-subxiphoid thymectomy which the whole operation can be completed by Da Vinci surgical system without the additional use of conventional thoracoscopy.
Methods
Patients
A total of 70 consecutive patients with myasthenia gravis or anterior mediastinal mass underwent robot-assisted trans-subxiphoid thymectomy during August 2016 and September 2017 in the Department of Thoracic Surgery, West China Hospital, Sichuan University. The operations were performed by a single surgical team experienced in both robotic surgery and trans-subxiphoid thoracoscopic thymectomy. The study protocol was approved by the applicable institutional review board. Written informed consents were obtained from all patients.
Patients who were preoperatively diagnosed with resectable thymic neoplasms or myasthenia gravis were included in this study. Exclusion criteria: (I) patients had impaired cardiac, kidney, liver or lung function and had a great risk; (II) preoperative CT scan revealed that patients with advanced tumors and the great vessels were invaded by the tumor and need reconstruction may require a median sternotomy; (III) the condition of the patients with myasthenia gravis was not stable; (IV) patients with irregular chest, such as funnel chest; (V) patients refused to undergo this surgical approach.
Surgical technique
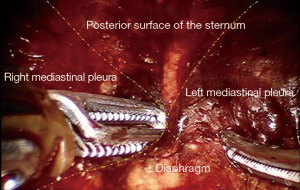
A 4-arm da Vinci Si robotic system (Intuitive Surgical, Inc., USA) was used for this operation. Under general anesthesia with two lung ventilations, the patient was placed in the reverse Trendelenburg position. Four incisions for introducing camera and instruments were created and assistant incision was unnecessary (Figure 1). A 2-cm longitudinal incision for camera was made below the xiphoid process, without the necessity to excise it. By using a finger through this incision, the lower part of mediastinal pleura was detached from the back of the sternum, and the extrapleural space was enlarged blindly. The Figure 2 shows the view of the extrapleural space, which was surrounded by posterior surface of the sternum, bilateral mediastinal pleura and diaphragm. Approximately 10 cm away from the subxiphoid incision, additional two operation holes were created below the bilateral costal arches, and two 8-mm robotic trocars were inserted into the extrapleural space under the guidance of a finger. The ports were docked to the patient cart which comes from the patient’s head. In order to enlarge the very small anterior mediastinal spaces between the pericardium and sternum, the camera arm can be slightly elevated to lift the lower part of the sternum. Due to the movement of instruments might be limited by the upper abdominal wall, operational arms can also be slightly elevated to improve their maneuverability.
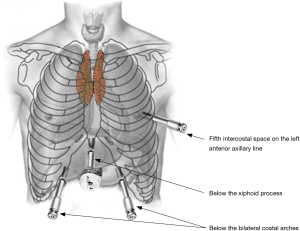
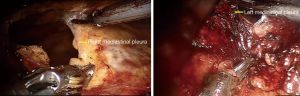
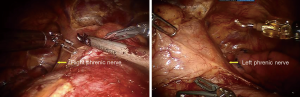
Carbon dioxide (CO2) was insufflated at a pressure of 8 mmHg, which maintain the lung and diaphragm off the surgical field to ensure a sufficient surgical field at the anterior mediastinum. Subsequently, ultrasonic scalpel and fenestrated bipolar forceps were introduced into the extrapleural space from two subcostal arch robot-working ports (Figure 2). After bilateral mediastinal pleura were dissected (Figure 3), sufficient surgical exposure can be achieved by the gravity of mediastinum and the pressure of CO2. Thereafter, bilateral phrenic nerves could be easily visualized (Figure 4). The dissection of thymus and surrounding adipose tissues began at the pericardiophrenic angle, along bilateral phrenic nerves, to the upper lobes of the thymus. For most of the patients, however, the ultrasonic scalpel inserted through subcostal arch is inaccessible to the cervical region. Therefore, a third robotic trocar was placed in the 5th intercostal space on the left anterior axillary line. Through this port, a Cadiere Forcep can be introduced to improve the exposure if necessary, or it was used to introduce an ultrasonic scalpel or a cautery hook when dissection proceeding cranially up to the plane of the left innominate vein. The thymus was carefully separated from the underlying left innominate vein. The thymic veins were mobilized and transected with the ultrasonic scalpel. We preferred endo-wristed cautery hook rather than ultrasonic scalpel during operation in the narrow cervical region. After exposure of the superior thymic horns and the inferior portion of the thyroid gland, the upper thymic lobes and surrounding fat tissues were dissected caudally. When the resection was completed, the specimen was removed through the subxiphoid incision. Haemostasis was checked, followed by insertion of a chest tube through one of the subcostal arch instrument incision.
Results
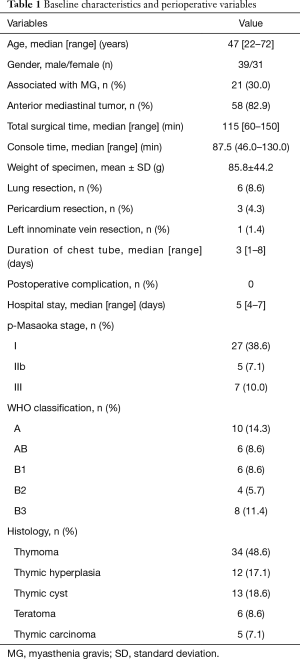
From August 2016 to September 2017, 70 consecutive patients (39 men and 31 women) met the inclusion and exclusion criteria and were successfully treated with this novel surgical procedure. Baseline characteristics and early surgical outcomes of the patients were shown in the table below (Table 1). The median age was 47 (range, 22–72) years. Twelve patients (17.1%) were preoperatively diagnosed of non-thymoma myasthenia gravis, 9 patients (12.9%) synchronous with myasthenia gravis and thymoma, and 58 patients (82.9%) with resectable thymic neoplasms. Because of tumor invasion, six patients synchronously underwent left lung wedge resection, three patients simultaneously received pericardium resection and one patient simultaneously underwent left innominate vein resection successfully without conversion. Median overall operative time was 115 (range, 60–150) min. Median console time was 87.5 (range, 46.0–130.0) min. The intraoperative blood loss was minimal for all the patients. Mean weight of resected specimen was 85.8±44.2 g. The median postoperative hospital stay was 5 (range, 4–7) days. The median duration of chest tube was 3 (range, 1–8) days. No patient required conversion to an open procedure, and there was no postoperative complication and in-hospital mortality. According to the postoperative pathological examination, there were no tumor residual in resection margin, and 34 patients diagnosed with thymoma (WHO classification: 10 type A; 6 type AB; 6 type B1; 4 type B2, 8 type B3), 12 patients with thymic hyperplasia, 13 patients with thymic cyst, six patients with teratoma and five patients with thymic carcinoma. Pathologic staging revealed Masaoka stage I in 27 patients, IIb in 5 patients and III in 7 patients. During the follow-up period, all the patients were uneventful, no tumor recurrence happened and the neurological outcomes of patients with myasthenia gravis remain stable.
Full table
Discussion
Despite the unilateral approach is the most preferred route for thoracoscopic thymectomy, this minimally invasive procedure can’t always provide a sufficient visualization of bilateral upper thymus lobes and the contralateral phrenic nerve. Conversely, the trans-subxiphoid thymectomy has several positive aspects and increasingly gained attention: it could provide the similar operation field as sternotomy; patients may experience less postoperative pain compared with the unilateral route, bilateral route or sternotomy; all the incisions that locate at the lower chest or the upper abdomen are cosmetic, especially for young patients; the subxiphoid incision benefits for removal of the specimen, especially for tumor of large size; the capsule of the tumor could be preserved without the obstruction of the narrow intercostal space, which is important for the precise pathological stage. However, the trans-subxiphoid thoracoscopic is a technically demanding operation associated with ergonomic discomfort. Herein, we introduce the da Vinci robotic system to facilitate this surgical procedure.
From our experience, the robotic surgical system provides considerable advantages over standard thoracoscopy in trans-subxiphoid thymectomy, including articulating surgical instruments benefit precise dissection in the confined anterior mediastinum and the three-dimensional, magnified operative images and tremor filtration minimizes the possibility of accidental injury to the surrounding vital structures. Additionally, operation at the surgeon console presented the surgeon a much more ergonomically comfortable position with minimum fatigue.
According to the literatures on trans-subxiphoid thoracoscopic thymectomy, the instruments can reach the working space in the anterior mediastinum via three different surgical approaches: through a single subxiphoid incision (3), below the bilateral costal arches (4) or in the bilateral intercostal space (5). In the only clinical report on trans-subxiphoid robotic thymectomy which can be retrieved in the PubMed, Suda et al. (2) chose bilateral sixth intercostal space to introduce the robotic instruments. The reason we speculated was to ensure the robotic instruments of limit length access to the whole anterior mediastinum and the lower cervical region. However, the camera and two instruments were located in three separated cavities in this operation mode at the beginning. Therefore, before the da Vinci robotic system can be used, a technically demanding and time-consuming single-port thoracoscopic operation must be performed to dissect the bilateral pleura in order to communicate the extrapleural space and bilateral pleural spaces.
In our modified procedure, the ports for robotic instruments were placed below bilateral costal arches. Therefore, the camera port and instrument ports could be located in the same extrapleural space created by finger blindly as described above and obviate the need of additional conventional thoracoscopy to dissect the mediastinal pleura. For most patients, the subcostal instruments were not long enough to reach the superior poles of the thymus, so we introduced a third robotic arm at the 5th intercostal space to dissect upper parts of the thymus.
During thoracoscopic thymectomy, it is important to enlarge the retrosternal space and ensure an adequate working space between the pericardium and sternum. This was usually achieved by providing downward press on the mediastinum and the pericardium in the conventional thoracoscopic thymectomy, which may result in arrhythmia or hypotension sometimes. Besides, some special equipment were also developed to lift the sternum and enlarge the retrosternal space (6). In our opinion, by moderately lifting the camera and instruments arms, the similar effect as a sternal retractor can be achieved conveniently in robotic thymectomy. According to the oral feedbacks of the anesthetists in our hospital, the haemodynamics characters were more stable in patients undergoing robotic thymectomy than patients received conventional trans-subxiphoid thoracoscopic thymectomy.
The time required to dock, undock, and exchange instruments is time-consuming in robotic surgery (7). However, the median total surgical time in our case series was not inferior to traditional trans-subxiphoid thoracoscopic thymectomy (8). In China, the high cost and relatively limited availability of the da Vinci surgical system might be the major reason for non-extensive usage of robotic thymectomy.
The opening of the bilateral pleural spaces increases the surgical exposure of the anterior mediastinum and could facilitate the tumor dissection. However, it might also increase the possibility of the diffusion of neoplastic cells in pleura and so the growth of pleural recurrences. In order to minimize the risk of tumor seeding, the surgeon should pay much attention to grasp the normal tissues but not touch the tumor capsule.
Although the number of patients is limited, the short-term outcomes indicated robot assisted thymectomy via the subxiphoid approach is safe and technically feasible. Excellent surgical exposures, precise dissection with dexterity and good ergonomics for the surgeon are the main advantages over standard trans-subxiphoid thoracoscopic thymectomy. But the long-term oncologic efficacy and the neurological outcome of this approach needs to be followed. Besides, these results are obtained in one center and only a small series, so a prospective multicenter long-term follow-up trial is required.
Acknowledgements
Funding: This study was supported by grants from Chengdu City Science and Technology Project of China (No. 0040205301E42) and the National Key Research Project of China (No. 2017YFC0113502).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the applicable institutional review board (No. 016-68). Written informed consents were obtained from all patients.
References
- Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg 2015;4:495-508. [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Zhao J, Wang J, Zhao Z, et al. Subxiphoid and subcostal arch thoracoscopic extended thymectomy: a safe and feasible minimally invasive procedure for selective stage III thymomas. J Thorac Dis 2016;8:S258-64. [PubMed]
- Hsu CP, Chuang CY, Hsu NY, et al. Subxiphoid approach for video-assisted thoracoscopic extended thymectomy in treating myasthenia gravis. Interact Cardiovasc Thorac Surg 2002;1:4-8. [Crossref] [PubMed]
- Bakker PF, Budde RP, Gründeman PF. Endoscopic robot-assisted extended thymectomy by subxiphoid approach with sternal lifting: feasibility in the pig. Surg Endosc 2004;18:986-9. [Crossref] [PubMed]
- Straughan DM, Fontaine JP, Toloza EM. Robotic-Assisted Videothoracoscopic Mediastinal Surgery. Cancer Control 2015;22:326-30. [Crossref] [PubMed]
- Yano M, Moriyama S, Haneda H, et al. The Subxiphoid Approach Leads to Less Invasive Thoracoscopic Thymectomy Than the Lateral Approach. World J Surg 2017;41:763-70. [Crossref] [PubMed]