Intratumoral heterogeneity of Notch1 expression in small cell lung cancer
During the tumorigenesis processes, cancer cells exhibit genetic and epigenetic changes, hierarchal tumor cell organization, and subclonal cell-cell interaction, which may cause more heterogeneous cancers (1,2). The heterogeneity can also be modified by therapies and microenvironments including hypoxia, acidity, inflammation, immunological responses, and extracellular matrix components (1,3). Analysis of tumor heterogeneity may help to resolve the subclonal origins of therapeutic resistance, relapsed diseases, and distant metastases, and mechanistic study and accurate assessment of tumor heterogeneity is crucial for effective therapies (1,2). Small cell lung cancer (SCLC) is a highly malignant neoplasm characterized by neuroendocrine differentiation, rapid tumor growth, high vascularity, early metastatic potential, high chemosensitivity at the first therapeutic trial, acquisition of chemoresistance, mutation of both TP53 and Rb1, and genome instability (4-6). The importance of various pathways, such as cell cycle regulation associated with TP53 and RB1, receptor-kinase signaling, transcriptional networks including SOX2, Notch signaling, and guidance molecule signaling, has been reported by comprehensive analyses of SCLC samples and cell lines (7-9). Histologically, SCLC has long been recognized as a classical type homogenous neoplasm, although morphological heterogeneity is evident in the combined type SCLC, in which non-SCLC components including adenocarcinoma, squamous cell carcinoma, or large cell carcinoma are present to various extents (10). Regarding the clinical course, SCLC is considered to be a homogenous cancer because most patients of SCLC show initially high sensitivity to cytotoxic chemotherapy, but almost all tumors recur and acquire resistance to further therapy (4-6). Even though no discrete morphological heterogeneity is present in classical type SCLC tissue stained with hematoxylin and eosin, heterogeneity in biological, molecular, and clinical differences may occur in cancer cells through various molecular mechanisms, and the tumor heterogeneity of SCLC has recently begun to gain attention. Heterogeneity in growth characteristics, epithelial-mesenchymal transition (EMT), master transcription factors for neuroendocrine differentiation, MYC family members, Notch, hedgehog and Wnt signaling pathways, and Sox2 related cancer cell pluripotency between SCLCs has been reported in different patients and cell lines (6,8,9,11-13).
In lung cancers, Notch exhibits both tumor-promoting and -suppressive functions depending on the histological type (14). Notch signaling is an essential cell signaling system for regulating differentiation, metabolism, cell cycle progression, angiogenesis, and stemness of cancer cells (15). Notch receptors 1–4 interact with ligands, such as the Delta and/or Jagged families, and induce several genes such as hairy/enhancer of split 1 (Hes1), cyclinD1, c-Myc, and Akt (16). A whole genome sequencing study of SCLC cases revealed mutations of Notch family genes in about 25% of the cases examined, suggesting the tumor-suppressive nature of Notch signaling in SCLC cells (9). Additionally, some classical SCLC cell lines with neuroendocrine differentiation express neuroendocrine transcription factors, such as Achaete-scute complex homologue 1 (Ascl1) and insulinoma-associated protein 1 (INSM1), and show an absence of Notch1 (13,14,17). Decreased expression of Notch1 may be regulated by histone modifications (18). In SCLC cell lines, gene transfection and knockdown experiments reveal that Notch1 plays an important role in the suppression of cell proliferation, enhancement of apoptosis, induction of epithelial morphology (mesenchymal-to-epithelial transition), suppression of motility, acquisition of drug resistance, and suppression of neuroendocrine differentiation (13,14,18). Regarding cell fate determination, the Notch1-Hes1 pathway represses neuroendocrine differentiation through decreased expression of neuroendocrine-promoting transcription factors such as Ascl1 and INSM1 (13,14,17,19-21). Mouse pulmonary neuroendocrine cells are positive for Ascl1, but negative for both Notch receptors and Hes1, while pulmonary non-neuroendocrine cells are negative for Ascl1 and INSM1, but positive for Notch receptors and Hes1 (19). This mutually exclusive expression pattern is true in almost all lung cancers, and most SCLC cases are positive for Ascl1 and/or INSM1, but negative for Notch1, while non-SCLC cases are negative for Ascl1 and/or INSM1, but positive for Notch1 (13,17). In addition to the major SCLC population with expression of Ascl1/INSM1, a NeuroD1-positive subpopulation has also been reported for SCLC (6,11). In the mouse pulmonary epithelium, NeuroD may be regulated by Notch signaling (19).
Recently, Lim et al. (22) reported the role of Notch signaling in heterogeneity in human and mouse SCLC. In a p53(flox/flox), Rb1(flox/flox), and p130(flox/flox) conditional triple knockout (TKO) mouse, mouse SCLC resembles that in humans, and the TKO mouse is a valuable pre-clinical model to identify novel therapeutic targets against SCLC (23). Crossing the above-mentioned genetically engineered mouse model (GEMM) of SCLC with a promoter Hes1-green fluorescent protein (GFP) reporter mouse is an effective method of monitoring Notch signaling activity, as Hes1 is a major transcriptional target molecule of Notch signaling. In this reporter GEMM mouse, GFP(−) indicated a low level of Hes1 expression and suggests inactive Notch signaling, while GFP(+) indicates a high level of Hes1 expression and suggests active Notch signaling (22). GFP(+) and Notch-active SCLC cells are non-neuroendocrine and slow-growing, consistent with a tumor-suppressive role for Notch. However, these cells are chemoresistant and can support growth of GFP(−) and Notch-inactive SCLC cells with neuroendocrine differentiation, consistent with a tumorigenic role for Notch as GFP(+) and GFP(−) cells interact with each other as in stromal-tumor interaction (22). These observations suggest the necessity for combined chemotherapies targeted both for GFP(−), Notch-inactive, and neuroendocrine SCLC cells, and for GFP(+), Notch-active, non-neuroendocrine SCLC cells to overcome intratumor heterogeneity (22). The article by Lim et al. (22) is very important and reports that Notch signaling can drive intratumor heterogeneity in SCLC and may be a target to overcome SCLC. Immunohistochemical studies of Notch1 in surgically resected SCLC tissue samples showed that most cases of SCLC were negative for Notch1, but that Hes1 was sometimes positively stained (unpublished observation). Additionally, Hes1 was detected in the classical SCLC cell lines with neuroendocrine features and negative Notch receptors (17). This suggests that Hes1 is not always regulated by Notch signaling and not all Hes1-positive cells exhibit active Notch signaling status.
Intratumor heterogeneity of SCLC in some reports may be explained by Notch signaling. For example, tumor cell heterogeneity is also observed in other mouse models of SCLC, in which the tumor cells are often composed of phenotypically different cells with either neuroendocrine or a mesenchymal marker profiles (24). It is hypothesized that the neuroendocrine tumor cells may be in an inactive Notch condition and the tumor cells positive for mesenchymal marker in an active Notch condition. In human SCLC, an INSM1-positive and YAP1-negative subpopulation and an INSM1-negative and YAP1-positive subpopulation have recently been reported (25). INSM1 expression in SCLC is negatively regulated by Notch signaling (17) and the INSM1-negative and YAP1-positive subpopulation of SCLC suggests the presence of an active Notch signaling condition in the population. The molecular mechanisms of the combined type SCLC have long been unknown; however, Notch signaling may drive this variant type SCLC. Transfection of the Notch1 gene or induction of Notch1 by histone modification in classical SCLC cell lines induced non-small cell carcinoma components when inoculated in immune-deficiency mice (13,14,17,18). One of the molecular mechanisms responsible for the combined type SCLC may be related to Notch signaling, which may be inactivated by mutations of Notch signaling-related genes (9) and suppression of Notch expression by histone deacetylation (18). Thus, Notch signaling is a driving force for the heterogeneity in SCLC and should be regulated by various techniques.
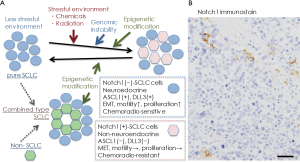
In conclusion, the significance of Notch signaling in producing intratumor heterogeneity has been reported in SCLC, and as the heterogeneity may be related to cancer progression, resistance to therapy, and disease relapse (1,6), SCLC cells with active Notch signaling may be a targeted therapeutically with Notch inhibitors in combination with cytotoxic chemotherapy (22). Considering the natural history of human SCLC, classical SCLC cells, Notch-negative and Ascl1/INSM1-positive, with neuroendocrine differentiation are very vulnerable to cytotoxic chemotherapy at the initial treatment, and then, in some cases, epigenetics mechanisms induce Notch1 induction in residual SCLC cells, which may lead to recurrence (Figure 1A). This idea is supported by Figure 1B, in which Notch1-positive SCLC cells recurred in cancer tissue of a patient after repeated chemotherapy.
Acknowledgements
I thank Ms. Motoko Kagayama and Mr. Shinji Kudoh for their skillful technical assistance. This study was supported in part by a grant from the Smoking Research Foundation.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018;15:81-94. [Crossref] [PubMed]
- Gupta RG, Somer RA. Intratumor Heterogeneity: Novel Approaches for Resolving Genomic Architecture and Clonal Evolution. Mol Cancer Res 2017;15:1127-37. [Crossref] [PubMed]
- Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 2012;12:323-34. [Crossref] [PubMed]
- Bunn PA Jr, Minna JD, Augustyn A, et al. Small Cell Lung Cancer: Can Recent Advances in Biology and Molecular Biology Be Translated into Improved Outcomes? J Thorac Oncol 2016;11:453-74. [Crossref] [PubMed]
- Sabari JK, Lok BH, Laird JH, et al. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol 2017;14:549-61. [Crossref] [PubMed]
- Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017;17:725-37. [Crossref] [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-42. [Crossref] [PubMed]
- Borromeo MD, Savage TK, Kollipara RK, et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep 2016;16:1259-72. [Crossref] [PubMed]
- Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015;12:445-64. [Crossref] [PubMed]
- Ito T, Kudoh S, Ichimura T, et al. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: significance of inactive Notch signaling and expression of achaete-scute complex homologue 1. Hum Cell 2017;30:1-10. [Crossref] [PubMed]
- Wael H, Yoshida R, Kudoh S, et al. Notch1 signaling controls cell proliferation, apoptosis and differentiation in lung carcinoma. Lung Cancer 2014;85:131-40. [Crossref] [PubMed]
- Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev 2007;17:52-9. [Crossref] [PubMed]
- Rizzo P, Osipo C, Foreman K, et al. Rational targeting of Notch signaling in cancer. Oncogene 2008;27:5124-31. [Crossref] [PubMed]
- Fujino K, Motooka Y, Hassan WA, et al. Insulinoma-Associated Protein 1 Is a Crucial Regulator of Neuroendocrine Differentiation in Lung Cancer. Am J Pathol 2015;185:3164-77. [Crossref] [PubMed]
- Hassan WA, Takebayashi SI, Abdalla MOA, et al. Correlation between histone acetylation and expression of Notch1 in human lung carcinoma and its possible role in combined small-cell lung carcinoma. Lab Invest 2017;97:913-21. [Crossref] [PubMed]
- Ito T, Udaka N, Yazawa T, et al. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 2000;127:3913-21. [PubMed]
- Ball DW. Achaete-scute homolog-1 and Notch in lung neuroendocrine development and cancer. Cancer Lett 2004;204:159-69. [Crossref] [PubMed]
- Morimoto M, Nishinakamura R, Saga Y, et al. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 2012;139:4365-73. [Crossref] [PubMed]
- Lim JS, Ibaseta A, Fischer MM, et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017;545:360-64. [Crossref] [PubMed]
- Schaffer BE, Park KS, Yiu G, et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res 2010;70:3877-83. [Crossref] [PubMed]
- Calbo J, van Montfort E, Proost N, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011;19:244-56. [Crossref] [PubMed]
- McColl K, Wildey G, Sakre N, et al. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer. Oncotarget 2017;8:73745-56. [Crossref] [PubMed]


