Lipoxin A4 regulates PM2.5-induced severe allergic asthma in mice via the Th1/Th2 balance of group 2 innate lymphoid cells
Introduction
Allergic asthma is characterized by a significant eosinophilic airway inflammation, an airway hyperreactivity, and a chronic T helper-2 (Th2) cell-type of immune response to various allergens, such as house dust mites, airborne particulates, or even animal dander (1-3). It is estimated that asthma, a typical chronic disease, affects approximately 300 million people worldwide so far (4). A type 2 immune response is responsible for most allergen-induced inflammation at mucosal surfaces and it is reflected in an overproduction of various cytokines secreted by Th2 cells (5). The body is very sensitive to the first stimulus for special allergens, which can stimulate naive CD4+ T cells to differentiate into Th2 cells (6). At the same time, TH2 cells bring the memory tag of those special allergens naturally. And then, the re-exposure of sensitized individuals to the same special allergens causes a violent stimulation of memory Th2 cells, which resulted in strong inflammatory reaction, with secreting the corresponding effector cytokines interleukin-4 (IL-4), IL-5, IL-9 and IL-13, resulting in inflammation (2,7).
Group 2 innate lymphoid cells (ILC2s) are a newly discovered member of the native lymphocyte family in recent years and play a vital role in regulating the Th2 immune response via regulating the Th1/Th2 balance (8). In the process of allergic reactions, ILC2s are sensitive to various allergens. They are not directly recognized allergens but stimulated by cytokines including IL-33 released by damaged epithelium (9). In response to allergens, ILC2s produce Th2 cell type cytokines inducing T cell-independent allergic inflammation. Whereas, the crucial role of Th2 cells in allergic inflammation is well confirmed, ILC2s are also crucial sources of numerous cytokines, the role of which is equally of importance (10). It is generally thought that IL-4 in particular is believed to be critical for Th2 cell differentiation, as well as binding to its receptor activates signal transduction and transcriptional activation factor 6 (STAT6), which induces the expression of key transcription factor retinoid acid receptor related orphan receptor α (RORα) and GATA3, it also drives the production of type-2 cytokines (11-13). However, it is no clear that the initial source of IL-4 responsible for the differentiation of naive CD4+ T cells into Th2 cells because of complex cell population sources, such as natural killer T (NKT) cells, γσT cells, basophils, dendritic cells (DCs) and naive CD4+ T cells (14). Hence, the specific mechanisms by which allergens initiate the differentiation of naive CD4+ T cells into Th2 cells during the anaphylactic reaction are not well understood.
With the attention of the world to the ecological environment pollution, PM2.5 as a new type of allergens is gradually well known, which refers to the atmospheric aerodynamic equivalent diameter less than or equal to 2.5 micron particles (15,16). In recent years, many cases of asthma are reported to be induced by PM2.5. Epidemiological studies indicate that air pollution, especially elevated concentrations of PM2.5 microns is one of the major factors responsible for the increased incidence of asthma (17). Therefore, most countries around the world have become aware of the potential dangers of PM2.5 and have formulated testing standards to closely monitor PM2.5 concentration to ensure citizens’ health. For example, judgment standard of PM2.5 from the World Health Organization (WHO) is 10 µg/m3 (annual average concentration) and 25 µg/m3 (daily average concentration) (18). LXA4 was as an important anti-inflammatory mediator to regulate inflammation and was known as the inflammatory response of the “brake signal” (19). But there is rare to report on LXA4 in the research of asthma treatment.
Thus, we speculated that when the human body is exposed to fine particles, PM2.5 can directly stimulate the natural immune cells such as alveolar macrophages to increase the secretion of IL-33, induce the aggregation and activation of lung ILC2s, promote the occurrence and development of airway inflammation and airway hyperreactivity, of which effect is mainly through the cytokines of Th2 cells to achieve. In this study, the severe allergic asthma mice of PM2.5-induced were selected as the research object. At the same time of PM2.5 induction, using LXA4 complementary therapy. At the end of modeling and treatment, the changes of related indicator in bronchial alveolar lavage fluid (BALF), peripheral blood mononuclear cells (PBMC) and lung tissue were measured. Our findings suggest that ILC2s are probably responsible for the sensitization of individuals to multiple allergens via the Th1/Th2 imbalance. Surprisingly, LXA4 has a good effect on Th1/Th2 balance in allergic asthma.
Methods
Animals and ethics statement
Forty male specific pathogen free (SPF) BALB/c mice (8–10 weeks old, 20±2 g) were purchased from Hubei Province Center for Disease Control and Prevention (Wuhan, China). Mice were kept in a specific pathogen-free environment with a 12 h light/dark cycle and provided with water and food ad libitum everday. All mice were acclimated to laboratory conditions for 1 week before modeling. All animal experiments were performed in accordance with the guidelines and with the approval of the Medical Ethics Committee of Wuhan Medical & Healthcare Center for Women and Children (number of the approval: 2016050).
Main reagents of modeling
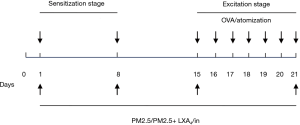
OVA (A5503, Sigma, USA), Al(OH)3 (A137846, Aladdin, Shanghai, China), PM2.5 particles (Hubei Province Center for Disease Control and Prevention, Wuhan, China). Preparing methods of PM2.5 (provided by the Hubei Province Center for Disease Control and Prevention): first, PM2.5 was collected on a glass fiber filter using a Thermon Anderson high-volume sampler (Thermon Anderson, San Marcos, Texas, USA). Then, the PM2.5 adsorbed membrane was cut into 1×3 cm2 size and immersed in ultrapure water for eluting PM2.5, shocked 30 min at low temperature, 3 times. Finally, the eluted PM2.5 with gauze filtration, the filtrate was vacuum freeze-dried, −20 °C stored for future use or sale. The concentration of each reagent under the experimental conditions is as follows: sensitizer: 0.04 g/L OVA +0.2 g/L Al(OH)3, excitation liquid: 1 g/L OVA, PM2.5 suspension: 15 g/L PM2.5 (diluted by sterilized water), therapeutic: 0.5 mg/L LXA4 (Figure 1).
Model of asthma
A time-consuming 21-day asthma mouse model was constructed (Figure 2). Forty male SPF BALB/c mice were randomly divided into four equal groups: (A) control group (n=10); (B) OVA group (n=10); (C) OVA + PM2.5 group (n=10); (D) OVA + PM2.5 + LXA4 group (n=10). The modeling was carried out in two stages, the sensitization stage and the excitation stage. Sensitization stage: a total of 2 times, mice were injected 0.5 mL sensitizer with intraperitoneal injection on the 1st and 8th day, respectively. Excitation stage: from the 15th day, a total of 6 times, the mice were placed in a self-made closed atomizer and given the excitation liquid atomization 30 min. Among them, the two stages of normal control group were treated with physiological saline; OVA group was performed according to the normal modeling procedure; at the same time as OVA modeling, the mice in OVA + PM2.5 group were intranasally treated with 25 mL/kg ultrasonic oscillatory PM2.5 suspension (days 1, 8, 15 and 21, respectively); for OVA + PM2.5 + LXA4 group, 0.5 mL LXA4 treatment was given to each mouse on the basis of group OVA + PM2.5. All mice were anaesthetized with 1% pentobarbital sodium (1 mL/100 g) 21 days later for collection of blood, BALF and lung tissues.
BALF collection and cell counts
BALF was collected with 1.0 mL of saline after death using a tracheal cannula from a subset of 10 mice from each experimental group. After centrifuged (1,000 r/min) for 10 min, cell pellets were resuspended in 1 mL of phosphate buffered solution (PBS), and total BALF cell counts were determined with a hemocytometer. BALF cytospins were prepared, slides were fixed in acetone and then Wright-Giemsa staining was performed. The percentage of eosinophils in BALF on each slide was counted by counting a minimum of 200 cells with an oil immersion microscope.
Measurement of cytokines in BALF
Levels of IL-5, IL-9, IL-13 and IL-33 were measured by using enzyme-linked immunosorbent assay (ELISA) kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) under the manufacturer’s instructions.
Collection and microscopic examination of PBMC
The collected anticoagulated blood was diluted with an equal volume of PBS, then an equal volume of lymphocyte separation medium was added, 2,500 rpm, room temperature, centrifuge 30 min. After centrifugation, the liquid was divided into four layers, the upper layer was the plasma layer (after dilution), the middle layer was the separation liquid, the bottom layer was red blood cells and granulocytes, and the thinner mononuclear cell layer of milky white turbidity could be seen at the upper and middle liquid interface. A 1 mL syringe was used to aspirate the intermediate white film layer, and the lymphocyte separation solution was washed with 10 volumes of PBS and repeated twice. Finally, a small number of cells were taken to observe the morphology of the cells under a microscope for subsequent testing.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) of PBMC
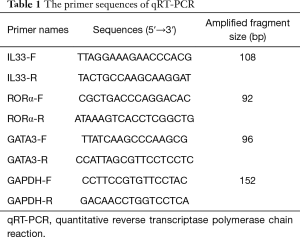
The expression levels of RORα, GATA3 and IL-33 mRNA in PBMC were detected by qRT-PCR. Relative expression levels were calculated by the 2-△△Ct method of using the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an endogenous control for normalization. Ribonucleic acid (RNA) extraction (Trizol method), and reverse transcription (Takara, Japan) were followed by the instructions. SYBR Green I was purchased from Life Technologies (AB & Invitrogen). All primers were synthesized by Sangon (Shanghai, China) and shown in Table 1.
Full table
Flow cytometry for analysis of the Th2/Th1 ratio in PBMC
The primed CD4+ T cells in PBMC were blocked for FcR by staining with anti-FcR (2.4G2) (BD Biosciences, San Diego, USA), followed by staining with biotin-anti-CD4 (BD Biosciences) in the presence of streptavidin-cychrome. The cells were then fixed with 4% paraformaldehyde and permeabilized with 0.1% saponin, followed by staining with FITC-anti-IL-4 and PE-anti- IFN-γ (BD Biosciences). The CD4+ T cells were gated and analyzed on a FACScanto II (BD Biosciences). Datas were displayed as bar graph of IFN-γ PE vs. IL-4 FITC. IFN-γ+ and IL-4− cells were defined as Th1 cells, while IFN-γ− and IL-4+ cells were deemed as Th2 cells. At last, the corresponding ratio of Th1/CD4+ cells, Th2/CD4+ cells and Th2/Th1 cells were calculated and analyzed.
Histological analysis of lung tissue
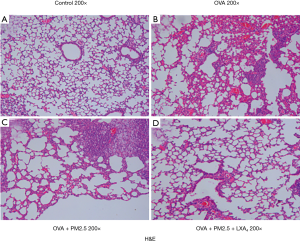
After anesthesia, the lungs of mice in each group were removed, and a part of lung tissues was fixed with 10% neutral phosphate-buffered formalin and embedded in paraffin. The fixed lung tissues were dehydrated embedded in paraffin and sectioned (5 µm thick). Hematoxylin and eosin (H&E) staining was used for the inflammatory changes of the lung tissue. Pathological changes were observed by light microscopy at 200× magnification.
Statistical analysis
All results were presented as the mean ± SD. Statistical analyses were performed using SPSS 19.0 (SPSS, Inc., Chicago, USA). T-test was used to compare the differences between two groups. Differences were considered statistically significant at P values less than 0.05. All histograms were drawn using GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA, USA).
Results
BALF cells count and analysis
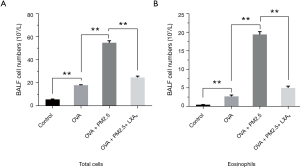
To evaluate the effects of the PM2.5 and LXA4 on lung inflammation induced by OVA, the cellular profile of BALF was investigated (Figure 3). Compared with the model group (Figure 3A), PM2.5 can significantly increase the total number of BALF cells in asthmatic mice induced by OVA (P<0.01). However, LXA4 can significantly decrease the total number of BALF cells in severe asthmatic mice caused by PM2.5 and OVA (P<0.01). For eosinophils (Figure 3B), the changes of cell numbers are same as that in the total number of cells. Take together, PM2.5 can increase the number of eosinophils and total cells of BALF, whereas the effect of LXA4 is just opposite.
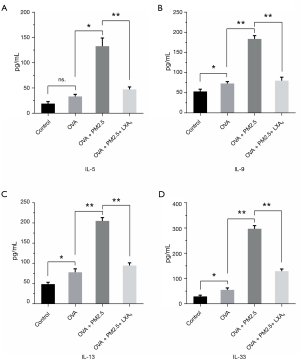
The changes of cytokines in BALF (ELISA)
To explore the influences of PM2.5 and LXA4 on cytokines in BALF, the levels of IL-5, IL-9, IL-13 and IL-33 were detected by ELISA according to the manufacturer’s protocol. The results of four cytokines can be secreted by Th2 cells, which are shown in Figure 4, compared with OVA group, the levels of IL-5, IL-9, IL-13 and IL-33 in OVA + PM2.5 group were significantly improved in varying degrees (P<0.05, P<0.01). Whereas, under the LXA4 treatment, the levels of four cytokines detected were significantly lower than those in the PM2.5 effects (P<0.01). In brief, PM2.5 promotes the secretion of IL-5, IL-9, IL-13 and IL-33 cytokines from Th2 cells, but LXA4 shows the inhibitory effect.
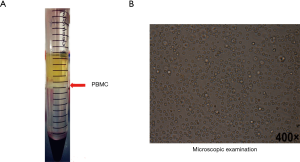
PBMC collection and microscopy
As shown in Figure 5, PBMC was successfully isolated from peripheral blood, just as shown in the milky white turbidity (Figure 5A). Microscopic observation shown cell morphology characteristics were significant and the cell recovery rate was higher (Figure 5B), of which provided fully credible materials for the subsequent testing.
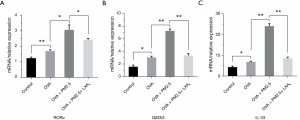
The mRNA level expression of RORα, GATA3 and IL-33 in PBMC
The mRNA expressions of RORα, GATA3 and IL-33 in PBMC were evaluated by PCR (Figure 6). In the group of PM2.5-induced severe asthmatic mice, RORα, GATA3 and IL-33 levels were increased compared with those in OVA-induced asthmatic mice. Nevertheless, in the group of LXA4 treatment, the corresponding expression levels of three targets were down-regulated observably (P<0.05, P<0.01). RORα and GATA3, as the vital transcription factor of ILC2s, the changes of which suggested that ILC2s play an important role in serious asthma induced by PM2.5. And the changes of IL-33 indicated that LXA4 may be related to the regulation of Th2/Th1 in treatment of asthma.
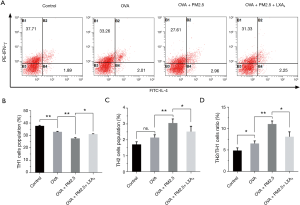
Measurement of Th2/Th1 ratio in PBMC
The Th2/Th1 ratio in PBMC was measured by flow cytometry and shown in Figure 7. The population (%) of Th1 cells was significantly reduced under the effect of PM2.5 (P<0.01), but increased under the LXA4 compared with PM2.5 induced (Figure 7A, P<0.05). On the contrary, the population (%) of Th2 cells was up-regulated under the effect of PM2.5 (P<0.01), but down-regulated under the LXA4 (Figure 7B, P<0.05). Thereupon, the variation trend of Th2/Th1 ratio was similar to the changes of population (%) of Th2 cells in PBMC (Figure 7C). All of which explained that maintaining the dynamic balance of Th2/Th1 is crucial for the treatment of asthma.
Effects of PM2.5 and LXA4 on histopathological analysis
Histopathological examinations revealed typical pathological features of asthma in OVA-induced asthmatic mice and PM2.5-induced severe asthma mice. As shown in Figure 8, the lung tissue of mice in the control group showed clear structures in the bronchus and alveoli with arranged neatly airway epithelial cells (Figure 8A). Compared with the control group, mice in OVA-induced asthmatic group showed disordered structures in the lung tissue, with widened alveolar septum, capillary dilatation, and a lot of inflammatory cells and neutrophil infiltration (Figure 8B). More seriously, under the induction of PM2.5, the alveolar septum was larger, the capillary dilation was more serious, and the infiltration of inflammatory cells was more obvious in lung tissue of mice (Figure 8C). Fortunately, there was a significant improvement in lesions of lung tissue in mice with LXA4 treatment compared with PM2.5 induction (Figure 8D). In short, the effect of LXA4 is very significant on improving the lung pathological state of mice.
Discussion
In this study, we investigated the effects of PM2.5 on asthma induced by OVA and the therapeutic effects of LXA4 on PM2.5-induced severe asthma. We found that PM2.5 exacerbated the changes in inflammatory cells in BALF, the degree of change in lung injury, as indicated by inflammatory cell infiltration, increased alveolar interval, and promoted capillary dilatation in asthma mice. In addition, the levels of Th2 cells-related cytokine secretion and expression of ILC2s-related transcription factors increased in varying degrees. On the contrary, LXA4 plays an excellent role in relieving PM2.5-induced asthma symptoms.
Due to the rising frequency of exposure to various environmental pollutants in air (20),the occurrence of asthma and allergic disorders is increasing in industrialized countries. The side effects of PM2.5 are drawing great attention in society. Numerous studies have demonstrated an exacerbating effect of PM2.5 in experimental asthma models (21-23). Besides, PM2.5 can act as an adjuvant for OVA to aggravate OVA-induced mice asthma symptoms (22). As a typical representative of airway high reactivity (AHR), the number of eosinophils in BALF and the expression of cytokines (IL-4, IL-13, and IL-1β) were increased significantly in allergic asthma model mice induced by PM2.5 collected from in Yokohama city (24). The study of the atmospheric environment proved that PM2.5 could elicit airway allergic reaction involving inflammasome mechanisms by the synergistic action of soluble factors and insoluble particles in NC/Nga mice (25). In the presence of OVA, PM2.5-rich dust caused severe eosinophil infiltration and goblet cell proliferation in airways, along with a marked induction of the Th2 cytokines IL-4 and IL-13, and the eosinophil-related cytokine IL-5 in BALF (26). And some reports indicated that asthmatic rats induced by OVA, of which exposed to concentrated air filled with PM2.5 particles had 200% increase in airway mucus and had more BALF neutrophils (250% increase), eosinophils (90%), and total protein (300%) compared to controls (27). All of these reports support our findings, adding to the evidence that PM2.5 does exacerbate asthma in animal models.
It is widely accepted that naive CD4+ T cells can differentiate into helper T cells such as Th1, Th2, Th9, Th17, and Th22 cells, and the Th1/Th2 imbalance plays an important role in the asthmatic airway (14,28,29). In the OVA exposure experiment, compared with control group, serum IL-4 levels were significantly higher in OVA-induced asthmatic mice, whereas serum IFN-γ levels and the IFN-γ/IL-4 ratio were obviously decreased, indicating that Th1/Th2 balance was disturbed in OVA-induced asthmatic mice (30). It is generally thought that cytokines, IL-12 and IL-4, are crucial for Th1 and Th2 cell differentiation, respectively (31). However, in IL-4-deficient mice, Th2 cells were efficiently generated upon helminth infection, and the same result also appeared in repeated intranasal injections of papain (32,33). Because ILC2s are known to be stimulated by both helminth infection and papain treatment, but that reaction does not secrete much IL-4, suggesting that ILC2s may be of great significance in an IL-4-independent pathway of Th2 cell differentiation.
However, how to maintain the balance of Th1/Th2 is the key to the treatment of inflammatory asthma. It is reported that astragalus membranaceus, a traditional Chinese herb plays an inhibitory role in airway inflammation in the model of asthma via modulating Th1/Th2 immune balance (34). Paeoniflorin, also can exert immunoregulatory effects to ameliorate asthma progression via regulating of the Th1/Th2 equilibrium (35). LXA4, an important inflammatory inhibitor, is still relatively rare for asthma treatment. The related research of LXA4 regulates NK cells and ILC2s activation in asthma, indicating that LXA4 decreased prostaglandin 2 (PGD2) and cytokine mediated IL-13 release by ILC2s and increased NK cells mediated eosinophil apoptosis (36). All of these results support the relevant conclusions of this study.
In conclusion, PM2.5 promotes the deterioration of OVA-mediated asthma mice symptoms by increasing inflammatory factors, highly expressed transcription factors associated with ILC2s, and poor lung pathological morphology. Nevertheless, LXA4 has a significant effect on improving the symptoms of malignant asthma mediated by PM2.5 via regulating the Th1/Th2 balance. Together, these findings assign critical roles to ILC2s in asthma pathobiology and identify new cellular targets and mechanisms. And anti-inflammatory and pro-resolving actions for LXA4 also suggest a potential new therapeutic strategy in asthma and allergic diseases that emphasizes this natural solution.
Acknowledgements
Funding: This research is supported by major project of the National Natural Science Foundation (No. 91643207), Nature Science Foundation of Hubei Province (No. 2016CFB663) and applied basic research plan of Wuhan science and technology bureau (No. 2015061701011632). Sincerely thank them.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All animal experiments were performed in accordance with the guidelines and with the approval of the Medical Ethics Committee of Wuhan Medical & Healthcare Center for Women and Children (number of the approval: 2016050).
References
- Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity 2009;31:412-24. [Crossref] [PubMed]
- Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 2010;11:577-84. [Crossref] [PubMed]
- Lund S, Walford HH, Doherty TA. Type 2 Innate Lymphoid Cells in Allergic Disease. Curr Immunol Rev 2013;9:214-21. [Crossref] [PubMed]
- Xu M, Xu J, Yang X. Asthma and risk of cardiovascular disease or all-cause mortality: a meta-analysis. Ann Saudi Med 2017;37:99-105. [Crossref] [PubMed]
- Marashian SM, Mortaz E, Jamaati HR, et al. Role of innate lymphoid cells in lung disease. Iran J Allergy Asthma Immunol 2015;14:346-60. [PubMed]
- Halim TY, Krauss RH, Sun AC, et al. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 2012;36:451-63. [Crossref] [PubMed]
- Miyauchi K. Helper T cell responses to respiratory viruses in the lung: development, virus suppression, and pathogenesis. Viral Immunol 2017;30:421-30. [Crossref] [PubMed]
- Kabata H, Moro K, Koyasu S, et al. Group 2 innate lymphoid cells and asthma. Allergol Int 2015;64:227-34. [Crossref] [PubMed]
- Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015;42:1005-19. [Crossref] [PubMed]
- Doherty TA, Khorram N, Lund S, et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol 2013;132:205-13. [Crossref] [PubMed]
- Kirstein F, Nieuwenhuizen NE, Jayakumar J, et al. Role of IL-4 receptor alpha-positive CD4(+) T cells in chronic airway hyperresponsiveness. J Allergy Clin Immunol 2016;137:1852-62. [Crossref] [PubMed]
- Weiss DL, Brown MA. Regulation of IL-4 production in mast cells: a paradigm for cell-type-specific gene expression. Immunol Rev 2001;179:35-47. [Crossref] [PubMed]
- Wong SH, Walker JA, Jolin HE, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol 2012;13:229-36. [Crossref] [PubMed]
- Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev 2013;252:12-23. [Crossref] [PubMed]
- Huang L, Pu Z, Li M, et al. Characterizing the indoor-outdoor relationship of fine particulate matter in non-heating season for urban residences in Beijing. PLoS One 2015;10:e0138559. [Crossref] [PubMed]
- Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol 2010;126:453-65. [Crossref] [PubMed]
- Chen Y, Wong GW, Li J. Environmental exposure and genetic predisposition as risk factors for asthma in China. Allergy Asthma Immunol Res 2016;8:92-100. [Crossref] [PubMed]
- Mukherjee A, Agrawal M. A global perspective of fine particulate matter pollution and its health effects. Rev Environ Contam Toxicol 2018;244:5-51. [Crossref] [PubMed]
- Qi W, Li H, Cai XH, et al. Lipoxin A4 activates alveolar epithelial sodium channel gamma via the microRNA-21/PTEN/AKT pathway in lipopolysaccharide-induced inflammatory lung injury. Lab Invest 2015;95:1258-68. [Crossref] [PubMed]
- Zhang J, Dai J, Yan L, et al. Air pollutants, climate, and the prevalence of pediatric asthma in urban areas of China. Biomed Res Int 2016;2016:2935163. [PubMed]
- Falcon-Rodriguez CI, De Vizcaya-Ruiz A, Rosas-Perez IA, et al. Inhalation of concentrated PM2.5 from Mexico City acts as an adjuvant in a guinea pig model of allergic asthma. Environ Pollut 2017;228:474-83. [Crossref] [PubMed]
- Liu H, Fan X, Wang N, et al. Exacerbating effects of PM2.5 in OVA-sensitized and challenged mice and the expression of TRPA1 and TRPV1 proteins in lungs. J Asthma 2017;54:807-7. [Crossref] [PubMed]
- Zhang X, Zhong W, Meng Q, et al. Ambient PM2.5 exposure exacerbates severity of allergic asthma in previously sensitized mice. J Asthma 2015;52:785-94. [PubMed]
- Ogino K, Nagaoka K, Okuda T, et al. PM2.5-induced airway inflammation and hyperresponsiveness in NC/Nga mice. Environ Toxicol 2017;32:1047-54. [Crossref] [PubMed]
- Ogino K, Takahashi N, Kubo M, et al. Inflammatory airway responses by nasal inoculation of suspended particulate matter in NC/Nga mice. Environ Toxicol 2014;29:642-54. [Crossref] [PubMed]
- He M, Ichinose T, Kobayashi M, et al. Differences in allergic inflammatory responses between urban PM2.5 and fine particle derived from desert-dust in murine lungs. Toxicol Appl Pharmacol 2016;297:41-55. [Crossref] [PubMed]
- Wagner JG, Morishita M, Keeler GJ, et al. Divergent effects of urban particulate air pollution on allergic airway responses in experimental asthma: a comparison of field exposure studies. Environ Health 2012;11:45. [Crossref] [PubMed]
- Ji NF, Xie YC, Zhang MS, et al. Ligustrazine corrects Th1/Th2 and Treg/Th17 imbalance in a mouse asthma model. Int Immunopharmacol 2014;21:76-81. [Crossref] [PubMed]
- Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol 2010;126:1081-91. [Crossref] [PubMed]
- Takahashi N, Saitoh T, Gotoh N, et al. The cytokine polymorphisms affecting Th1/Th2 increase the susceptibility to, and severity of, chronic ITP. BMC Immunol 2017;18:26. [Crossref] [PubMed]
- Yamane H, Paul WE. Cytokines of the gamma(c) family control CD4+ T cell differentiation and function. Nat Immunol 2012;13:1037-44. [Crossref] [PubMed]
- van Panhuys N, Tang SC, Prout M, et al. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc Natl Acad Sci U S A 2008;105:12423-8. [Crossref] [PubMed]
- Halim TY, Steer CA, Mathä L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014;40:425-35. [Crossref] [PubMed]
- Chen SM, Tsai YS, Lee SW, et al. Astragalus membranaceus modulates Th1/2 immune balance and activates PPARgamma in a murine asthma model. Biochem Cell Biol 2014;92:397-405. [Crossref] [PubMed]
- Zhang T, Yang Z, Yang S, et al. Immunoregulatory effects of paeoniflorin exerts anti-asthmatic effects via modulation of the Th1/Th2 equilibrium. Inflammation 2015;38:2017-25. [Crossref] [PubMed]
- Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med 2013;5:174ra26. [Crossref] [PubMed]