Age is major factor for predicting survival in patients with acute respiratory failure on extracorporeal membrane oxygenation: a Korean multicenter study
Introduction
Despite use of lung-protective ventilation strategies (1) and advanced adjunctive therapies (2) mortality of acute respiratory distress syndrome (ARDS) remains high at 40% (3). Since the 2009 H1N1 influenza pandemic, extracorporeal membrane oxygenation (ECMO) has emerged as a salvage therapy for severe ARDS (4,5), with a randomized controlled trial demonstrating a survival benefit from ECMO therapy (6). However, incremental hospital costs associated with ECMO (6), or complications such as bleeding (4,6) remain important issues. Therefore, appropriate patient selection is important prior to the initiation of ECMO. Several outcome prediction scoring systems have recently been developed, such as the ECMOnet score (7), the PRedicting dEath for SEvere ARDS on VV-ECMO (PRESERVE) score (8), and the Respiratory ECMO Survival Prediction (RESP) score (9). The RESP score is widely accepted survival prediction model at ECMO initiation for severe acute respiratory failure (ARF).
Overall hospital mortality rates were associated with increasing age (10) and the proportion of elderly patients in the intensive care unit (ICU) population is increasing (11). Mendiratta et al. reviewed patients older than 65 years treated with ECMO support in the Extracorporeal Life Support Organization (ELSO) registry between 1990 and May 2013. According to the study, the number of elderly patients receiving ECMO increased significantly recent years. Although survival at hospital discharge is low in elderly ECMO patients compared to the all adults, they emphasized that age should not be a firm contraindication for the initiation of ECMO (12).
In Korea, the use of ECMO in elderly patients is increasing also, and we wondered whether the RESP score could help to predict the survival in the population with large elderly patients. The aim of this study was to investigate the applicability of the RESP score in Korean cohort.
Methods
Study design
This retrospective multicenter study was conducted in ARF patients who did not respond to conventional treatment. From January 2014 to December 2015, patients who received ECMO therapy with acute respiratory and/or circulatory failure were included from 11 hospitals in Korea. This study was approved by the institutional review board of Asian Medical Center (approval No. 2016-0269), and each participating center approved the protocol. The requirement for informed consent was waived due to the retrospective design.
ECMO management
ECMO can provide both respiratory and circulatory support. VV ECMO to maintain gas exchange was primarily implemented for ARF patients. Veno-arterial (VA) ECMO was performed in patients with severe heart failure, hemodynamic instability, or pulmonary hypertension. Both types of ECMO involve inserting two cannulas following: one for draining the blood from venous system (superior vena cava/inferior vena cava) to ECMO circuit, the other one for returning the oxygenated blood either to right atrium (VV) or to arterial system (VA) (13). Cannulations were performed percutaneously. The standard configuration for VV ECMO was femoral vein and internal jugular vein, and femoral vein and femoral artery were preferred for VA ECMO.
Although indications for the use of ECMO have yet to be standardized between participating centers, decisions for ECMO initiation were based on the ELSO guidelines. ECMO therapy is recommended in patients with severe but potentially reversible respiratory failure with persistent hypoxemia or hypercapnia. According to the ELSO guidelines, ECMO is indicated when the risk of mortality is 80% or greater. This mortality risk is associated with a PaO2/FiO2 <100 on FiO2 >90% and/or a Murray score 3–4 despite optimal care for 6 hours or more. It can also be considered for patients with CO2 retention on mechanical ventilation despite high Pplat (>30 cmH2O). Relative contraindications for ECMO therapy were mechanical ventilation at high settings (FiO2 >0.9, Pplat >30) for 7 days or more, absolute neutrophil count <400/mm3, recent central nervous system hemorrhage, or terminal malignancy.
Data collection
Patients older than 19 years who received ECMO therapy in tertiary care centers were screened. After a review of electronic medical records, clinical data were recorded on the registry form. The ECMO registry form comprises baseline demographic data, ARF etiology, ventilation and hemodynamic parameters, and the results of arterial blood gas analysis (ABGA) prior to initiation of ECMO therapy. Any adjunctive therapy was also recorded, such as use of vasopressor, steroid, neuromuscular blockade, NO, polymyxin B-immobilized fiber column hemoperfusion, prone positioning, continuous renal replacement therapy (CRRT), and bicarbonate infusion. Acute physiology and chronic health evaluation (APACHE) II and sequential organ failure assessment (SOFA) scores were calculated using the worst value within 24 hours of ICU admission. Data for the ventilation and hemodynamic parameters, ABGA, and SOFA score were collected immediately, 4 hours, and 24 hours after ECMO cannulation. We also collected ECMO parameters including ECMO mode, equipment, membrane oxygenator, number of membrane changes, duration of ECMO support, and duration of mechanical ventilation prior to ECMO initiation. The primary outcome of the study was hospital survival and successful ECMO weaning (survival within 48 hours after weaning from ECMO) was recorded.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) Version 22.0 (IBM Corporation, Armonk, NY, USA), and differences with a P value <0.05 were considered statistically significant. Kolmogorov-Smirnov and Shapiro-Wilk tests for normal distribution were conducted. Continuous variables are reported as the mean ± standard deviation or median [interquartile range (IQR)]. Categorical variables are reported as numbers (percentages). For continuous variables, either a Student’s t-test or Mann-Whitney U test was performed depending on their distribution. For categorical variables, either a Chi-square test or the Fisher exact test was used to investigate comparisons between survivor and non-survivor groups. Discrimination of outcome prediction scores was evaluated by receiver operating characteristic (ROC) curve analysis. Sensitivity and specificity for the scores were determined and the cutoff point corresponded to the maximum of the Youden’s index. Univariate and multivariate logistic regression analyses were performed to identify the factors associated with hospital mortality. Variables with P<0.1 in the univariate analyses were included in the multivariate model and considered significant by forward stepwise selection at P<0.05.
Results
Baseline and clinical characteristics of the study population
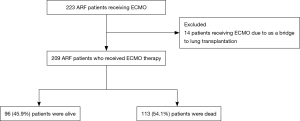
During the study period, 223 ARF patients received ECMO therapy. Among 14 patients (6.3%) who performed ECMO as a bridge to lung transplantation, 3 patients were underwent lung transplantation. After the exclusion of the 14 patients, 209 patients were analyzed (Figure 1). The successful weaning rate was 65.5% and 96 patients (45.9%) were alive at hospital discharge. The survival rates of VV ECMO and VA ECMO were 47.7% and 37.8%, respectively. Elderly patients older than 65 years were 51 with 31.4% of hospital survival.
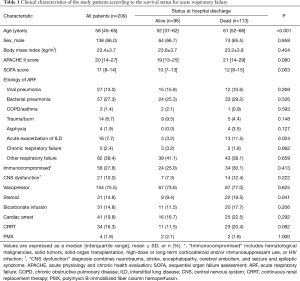
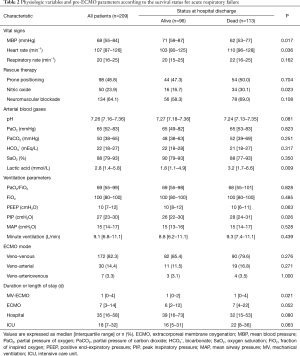
Data on baseline characteristics and pre-ECMO parameters are presented in Tables 1,2. In all patients, the median age was 58 (IQR, 45–65) years and pneumonia was the most common cause of ARF (40.2%). There were 58 (27.8%) immunocompromised patients and 31 patients (14.8%) were receiving steroids. Before ECMO initiation, the following rescue therapies were applied: prone positioning, 98 patients (48.8%); neuromuscular blockade, 134 patients (64.1%); and nitric oxide, 50 patients (23.9%). The median PaO2/FiO2 ratio was 69 (IQR, 55–99) mmHg with a high positive end-expiratory pressure level [10 (IQR, 7–12) cmH2O and peak inspiratory pressure level 27 (IQR, 23–30) cmH2O]. Pre-ECMO blood gases analyses showed that the PaO2 was 65 (IQR, 52–83) mmHg and the PaCO2 was 50 (IQR, 38–65) mmHg. VV ECMO was used for 82.3% of patients, and the median duration of ECMO support was 7 (IQR, 3–14) days.
Full table
Full table
Characteristics of survivors and non-survivors
The median age of survivors was lower than that of non-survivors [52 (IQR, 37–62) vs. 61 (IQR, 52–68) years, P<0.001]. There were no significant differences in sex, body mass index, and ARF etiology between survivors and non-survivors. Use of steroids and NO as a rescue therapy of ARF was significantly different between the two groups (9.4% vs. 19.5%, P<0.041; 16.7% vs. 30.1%, P=0.023). However, prone positioning, use of neuromuscular blockade, and bicarbonate infusion were not significantly different between the survivors and non-survivors.
Ventilator settings such as FiO2 and positive end-expiratory pressure were not significantly different, whereas peak inspiratory pressure was lower in survivors [26 (IQR, 22–30) vs. 28 (IQR, 24–31), P=0.026]. Before ECMO initiation, lactic acid, mean blood pressure, and heart rate were significantly different between the two groups: lactic acid [1.8 (IQR, 1.1–4.9) vs. 3.2 (IQR, 1.7–6.6), P=0.009], mean blood pressure [71 (IQR, 59–87) vs. 62 (IQR, 53–77), P=0.017], and heart rate [103 (IQR, 80–125) vs. 110 (IQR, 96–128), P=0.036].
Serial changes in the SOFA score and parameters after ECMO cannulation
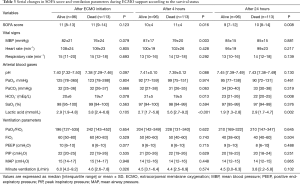
There were no differences between survivors and non-survivors in the initial lactic acid level, but it was lower in survivors after 4 hours [2.7 (IQR, 1.7–5.8) vs. 5.6 (IQR, 2.7–9.2), P<0.001; Table 3]. After 24 hours, the SOFA score was significantly different between survivors and non-survivors [9 (IQR, 7–12) vs. 10 (IQR, 8–14), P=0.008].
Full table
Predictors for hospital mortality in patients with ECMO support
In the logistic regression analyses, hospital mortality was associated with the following variables: age, APACHE II score, SOFA score, acute exacerbation of interstitial lung disease, CRRT, use of steroid, use of NO, positive end-expiratory pressure, peak inspiratory pressure, pH, and hospital stay before ECMO initiation. Multivariate analysis showed that age [odds ratio (OR) =1.044; 95% confidence interval (CI), 1.020–1.068; P<0.001], use of NO (OR =2.322; 95% CI, 1.045–5.161; P=0.039), and pH (OR =0.069; 95% CI, 0.008–0.625; P=0.017) were significant independent prognostic factors (Table 4).
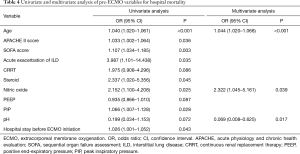
Full table
A comparison of the areas under the curves (AUCs) for pre-existing outcome prediction models is shown in Table 5. The AUC (c-statistic) of each outcome prediction score was as follows: RESP score, 0.66 (95% CI, 0.58–0.73); PRESERVE score, 0.63 (95% CI, 0.54–0.72); score proposed by Roch and colleagues, 0.56 (95% CI, 0.47–0.64); age, 0.67 (95% CI, 0.59–0.74); APACHE II score, 0.57 (95% CI, 0.49–0.64); and SOFA score, 0.62 (95% CI, 0.54–0.70). The optimal cutoff points for the RESP and PRESERVE scores were 0 (sensitivity of 75.6% and specificity of 47.6%) and 5 (sensitivity of 73.5% and specificity of 48.6%), respectively.

Full table
Modification of the RESP score
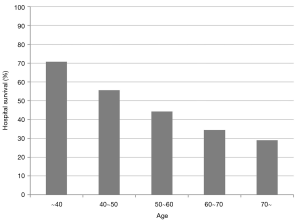
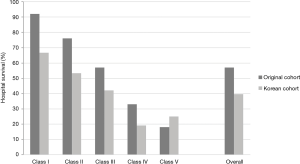
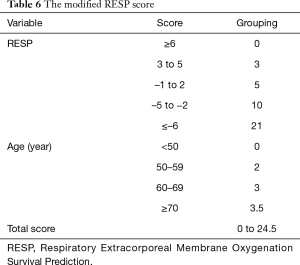
Survival rate declined with increasing patient age, and mortality was over 50% in patients over 50 years of age (Figure 2). The RESP score was calculated for our 209 patients and each risk class showed lower survival rate except for risk class V (Figure 3). Due to the discrepancy between the ELSO data and our cohort, we sought to develop for our cohort-specific prediction model. To improve the discriminative power of the RESP score, we added candidate variables that were independent risk factors according to multivariate logistic regression analysis. A final model was derived from the RESP score and reclassified age, and the predicted hospital survival according to the modified RESP score is described in Tables 6,7. Cumulative predicted hospital survivals were 70%, 57%, and 27 for the three risk classes, namely, I (0 to 3), II (4 to 7), and III (8 to 24.5), respectively. Internal validation of the modified RESP score exhibited reasonable discrimination [c=0. 71 (IQR, 0.63–0.78)] (Figure 4).
Full table
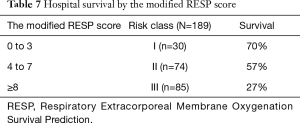
Full table
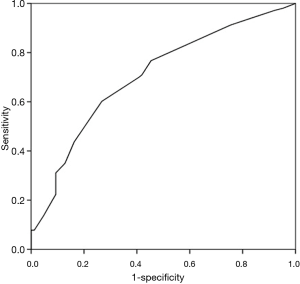
Discussion
This multicenter study involved a retrospective analysis of 209 patients receiving ECMO support for ARF refractory to conventional treatment and was conducted to investigate the applicability of the recently proposed outcome prediction models. Our result shows that the RESP score is significant model for predicting outcomes in a Korean ECMO population. In addition, age is an important factor in the survival of patients treated with ECMO. Age alone showed similar discrimination ability for prediction of mortality compared to the RESP score and elderly patients had higher mortality rate. Therefore, further studies are needed to guide decision making for ECMO initiation in elderly patients.
In our present study, we validated three outcome prediction-scoring models: the score by Roch and colleagues, the PRESERVE score, and the RESP score. The Roch score, which includes age, SOFA score, and influenza pneumonia, was developed to predict prognosis in ARDS patients who underwent cannulation in a distant hospital (14). Application of this prediction model in our cohort is difficult because only 8.1% of patients were transferred from a referring hospital and the ROC curve analysis showed no discriminative power in predicting hospital mortality in our data set (c=0.56).
Comparing our baseline characteristics to the PRESERVE study (8), the incidence of immunocompromised patients was similar, whereas the median SOFA score and the incidence of steroid use, NO inhalation, prone positioning, and CRRT were lower in our study population. The reason for this difference is that the PRESERVE and Roch scores were designed for pre-ECMO mortality prediction in patients with ARDS (a more specific population than ARF). The AUC of the PRESERVE score in our cohort demonstrated significant performance but weaker discriminative power than in the original data (c=0.63 vs. c=0.89). We postulate that the poor discriminatory ability of the PRESERVE score is because our population tended to be older with a lower incidence of prone positioning and body mass index, all variables of the score calculation associated with worse prognosis.
The RESP score (9), which was constructed from 2,355 ECMO-treated patients with severe ARF, was a discriminatory survival model (c=0.73). However, in our population, the RESP score also showed average discrimination ability to predict survival from ECMO therapy (c=0.66). This result is presumably due to the difference in the RESP score parameters between study populations. In other words, our cohort included older patients with a higher incidence of immunocompromised status and cardiac arrest than the RESP study. Recently, Klinzing et al. (15) performed external validation of these outcome prediction models on a dataset of 51 patients with severe ARDS. Interestingly, although there was no similarity between the study populations, the discrimination of each outcome prediction model was comparable (c=0.67 for the PRESERVE score, c=0.65 for the RESP score, and c=0.55 for the Roch score). They concluded that the PRESERVE and RESP scores were useful tools, particularly in the subgroup of patients who receive VV ECMO (c=0.75 and c=0.81, respectively). However, in our study, there was no better predictive ability in patients treated with VV ECMO. Therefore, we believe that modification of the RESP or PRESERVE scores for each study population would help to predict survival for ECMO therapy.
In the PRESERVE study (8), 60% of patients treated with ECMO were alive 6 months after ICU discharge, and the survival rate was at least 50% in most studies (4,6,7,9,15-17). According to the ELSO registry, the hospital survival rates of ECMO patients were 50.3% from 1986 to 2006 (16) and 56.8% from 2002 to 2012 (9). Despite this steady improvement in the survival rate and major technological advances in devices (18-21), our study showed a 45.9% survival rate. Mortality is influenced by pre-ECMO variables such as older age, organ dysfunction, immunocompromised status, and impaired lung compliance (21).
Many countries are faced with ageing populations and elderly patients consume a high proportion of intensive care (10,11). Although there was no specific age contraindication in the ELSO guideline, it is necessary to consider increasing risk with increasing age (22). Mendiratta et al. reported that survival rate of patients older than 65 years treated with ECMO was 41% (12), which was 10% higher compared with our cohort. However, they reviewed only 368 patients in the ELSO registry, which was including more than 40,000 patient-cases. Previous other studies demonstrated that age is an independent risk factor for survival in patients treated with ECMO (8,9,14,17). In the RESP study (9), age over 50 years was associated with increased mortality and the lowest score was assigned to patients aged 60 and over. However, we needed adjustment of the RESP score because 15% of our patients were aged 70 and over. Moreover, median age of 58 in our cohort was much higher than 41 years in the RESP study based on the ELSO registry. Therefore, reclassification of age group in the RESP score is convincing and the modified RESP score might be helpful for triage of ECMO initiation in the countries with the elderly population.
The SOFA score is a good prognostic model to assess organ dysfunction or failure over time and correlated with mortality in the ICU (23). Roch et al. (14) demonstrated that the SOFA score immediately before ECMO was an independent risk factor for mortality (OR 1.267, P=0.01), and Enger et al. (17) reported that the SOFA score showed better discrimination than the PRESERVE and ECMOnet scores. In accordance with previous studies, the SOFA score was a significant outcome prediction model in our analysis. Moreover, we speculated that serial evaluation of the SOFA score would be a good indicator of prognosis in patients on ECMO therapy because the SOFA scores at 24 hours after ECMO cannulation were significantly lower in survivors than in non-survivors.
Immunosuppression was associated with reduced functional reserves and mortality (8,9,17). Although the results of the present study did not correspond with those of previous studies, immunocompromised status is a valuable parameter used in both the PRESERVE and RESP scores. Another distinctive characteristic in our cohort was a higher incidence of non-VV cannulation. Non-VV cannulation was applied in our study in 17.7% of patients, whereas it was applied in 5% and 2% of patients in the PRESERVE and ECMOnet studies (7,8), respectively. Although non-VV cannulation was not a significant risk factor for mortality in our analysis, a number of studies showed that VA cannulated patients tended to have worse prognosis (15,16,24,25). Generally, non-VV cannulation was performed in patients with cardiogenic dysfunction or hemodynamic instability (7,8). In addition, the incidence of cardiac arrest in our study was higher than in the RESP study (19.8% vs. 9%) (9). Therefore, we postulated that non-VV cannulation was an indirect indicator of a poor outcome.
There were some limitations to our study of note. First, because this was a retrospective study and included only a Korean population, there is a limitation in terms of the general applicability of the results. Second, we failed to evaluate long-term outcomes because our study lacked data such as mortality at 6 months after ICU discharge or long-term quality of life. Third, external validation of the modified RESP score was not conducted due to the small study population. Thus far, the RESP score has been the most recommended outcome prediction model to identify specific populations who could benefit from ECMO therapy (21). Although further validation of our modified RESP score is necessary by other study populations, our study suggests that reclassification of age in the RESP score might be helpful.
In conclusion, the RESP score is significant model for predicting outcomes in a Korean ECMO population. Elderly patients had higher mortality, and age alone showed similar discrimination ability for prediction of mortality compared to the RESP score.
Acknowledgements
The authors thank study coordinators including Cho Eun Mi and Eun Shim for their support during data collection.
Funding: This study was supported by a grant of the Korea Health Technology R & D Project through the Korea Health industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1507).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Asan Medical Center, Samsung Medical Center, Pusan National University Yangsan Hospital, Seoul National University Bundang Hospital, Hallym University Sacred Heart Hospital, Chonbuk National University Hospital, Ulsan University Hospital, Bundang CHA Hospital, Kyung Hee University Hospital at Gangdong, Dongguk University Ilsan Hospital, and Hallym University Kangnam Sacred Heart Hospital. The need for informed consent was waived due to the retrospective design.
References
- Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Diaz JV, Brower R, Calfee CS, et al. Therapeutic strategies for severe acute lung injury. Crit Care Med 2010;38:1644-50. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Pham T, Combes A, Roze H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013;187:276-85. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med 2013;39:275-81. [Crossref] [PubMed]
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- Andersen FH, Kvale R. Do elderly intensive care unit patients receive less intensive care treatment and have higher mortality? Acta Anaesthesiol Scand 2012;56:1298-305. [Crossref] [PubMed]
- Flaatten H, de Lange DW, Artigas A, et al. The status of intensive care medicine research and a future agenda for very old patients in the ICU. Intensive Care Med 2017;43:1319-28. [Crossref] [PubMed]
- Mendiratta P, Tang X, Collins RT 2nd, et al. Extracorporeal membrane oxygenation for respiratory failure in the elderly: a review of the Extracorporeal Life Support Organization registry. Asaio J 2014;60:385-90. [Crossref] [PubMed]
- Pavlushkov E, Berman M, Valchanov K. Cannulation techniques for extracorporeal life support. Ann Transl Med 2017;5:70. [Crossref] [PubMed]
- Roch A, Hraiech S, Masson E, et al. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med 2014;40:74-83. [Crossref] [PubMed]
- Klinzing S, Wenger U, Steiger P, et al. External validation of scores proposed for estimation of survival probability of patients with severe adult respiratory distress syndrome undergoing extracorporeal membrane oxygenation therapy: a retrospective study. Crit Care 2015;19:142. [Crossref] [PubMed]
- Brogan TV, Thiagarajan RR, Rycus PT, et al. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med 2009;35:2105-14. [Crossref] [PubMed]
- Enger T, Philipp A, Videm V, et al. Prediction of mortality in adult patients with severe acute lung failure receiving veno-venous extracorporeal membrane oxygenation: a prospective observational study. Crit Care 2014;18:R67. [Crossref] [PubMed]
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. [Crossref] [PubMed]
- Gattinoni L, Carlesso E, Langer T. Clinical review: Extracorporeal membrane oxygenation. Crit Care 2011;15:243. [Crossref] [PubMed]
- Rozencwajg S, Pilcher D, Combes A, et al. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care 2016;20:392. [Crossref] [PubMed]
- ELSO Guidelines for Adult Respiratory Failure v1.4. August 2017. Available online: https://www.elso.org/Resources/Guidelines.aspx
- Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754-8. [Crossref] [PubMed]
- Hemmila MR, Rowe SA, Boules TN, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg 2004;240:595-605; discussion -7.
- Lee S, Yeo HJ, Yoon SH, et al. Validity of Outcome Prediction Scoring Systems in Korean Patients with Severe Adult Respiratory Distress Syndrome Receiving Extracorporeal Membrane Oxygenation Therapy. J Korean Med Sci 2016;31:932-8. [Crossref] [PubMed]


