Cerebral protection devices in transcatheter aortic valve replacement: a clinical meta-analysis of randomized controlled trials
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as an alternative to conventional aortic valve replacement (CAVR) for the treatment of severe aortic stenosis, particularly in patients of high or prohibitive surgical risk (1,2). Despite excellent short-term term efficacy outcomes to date for TAVR, the associated risk of stroke remains a concern with this procedure (1-6). Stroke is the most severe complication of TAVR, being associated with high morbidity and mortality (7).
There is increasing evidence that patients undergoing TAVR procedures have not only increased risk of neurological events but also silent cerebral lesions, which can be detected using diffusion weighted magnetic resonance imaging (DW-MRI). Although there is currently no evidence to inform clinicians of the long-term sequelae of these initially silent lesions, there have been reports of associations with poor functional outcome including memory loss and cognitive decline (3,8-10). As such, the advent of cerebral protection (CP) devices to reduce the risk of stroke and silent cerebral lesions associated with TAVR has drawn great interest. Although the results from several studies have suggested that CP may reduce cerebral infarction markers or improve early cognition, these parameters are ultimately surrogate markers for neurological events, and indeed there has been no conclusive clear benefit demonstrated by CP devices on hard clinical endpoints (11-14). The largest available randomized controlled trial (RCT) was the recent SENTINEL trial, which included 363 patients and showed that TAVR + CP was safe, captured embolic debris in 99% of patients but did not reduce major adverse cardiac and cardiovascular events (12).
The present systematic review and meta-analysis aims to compare outcomes of TAVR + CP and TAVR alone. The primary endpoint is the composite endpoint of all-cause mortality and stroke at 30 days, with additional endpoints of all-cause mortality, stroke, life threatening bleed, acute kidney injury, major vascular complications and changes in total lesion brain volume on MRI scan.
Methods
Literature search strategy
The present systematic review and meta-analysis followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club and Database of Abstracts of Review of Effectiveness (DARE) from their dates of inception to May 2017. To achieve maximum sensitivity of the search strategy and identify all studies, we combined the terms: “TAVR”, “embolic protection”, “cerebral protection”, “TAVR stroke”, “Claret”, “Embol-X”, “Triguard”, as either keywords or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria.
Selection criteria
Eligible RCTs for the present systematic review and meta-analysis included those in which patient cohorts underwent TAVR procedures with or without CP. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded. Review articles were omitted because of potential publication bias and duplication of results.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each retrieved article. Discrepancies between the two reviewers were resolved by discussion and consensus. Assessment of risk of bias for each selected study was performed according to the most updated Cochrane statement. Variables extracted included 30-day mortality, 30-day stroke, life threatening or major bleeding, acute kidney injury, major vascular complications, new total lesion volume on MRI and proportion of patients with new brain lesions on MRI. Stroke was defined according to the valve academic research consortium-2 (VARC-2). The time point for MRI was between 2 and 7 days post TAVR. Major vascular complications was defined as any aortic dissection, aortic rupture, annulus rupture, left ventricle perforation, new apical aneurysm/pseudo-aneurysm, access site or access-related vascular injury.
Statistical analysis
The odds ratio (OR), weighted mean difference and standardized mean difference were used as summary statistics. In the present study, both fixed- and random-effect models were tested. In the fixed-effects model, it was assumed that treatment effect in each study was the same, whereas in a random-effects model, it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. I2 can be calculated as: I2 = 100% × (Q − df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom. If there was substantial heterogeneity I2>50%, the results of the random-effects model were presented to take into account the possible clinical diversity and methodological variation between studies. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.3.2 (Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature search
A total of 341 studies were identified after exclusion of non-English, non-human studies, case reports, review articles and commentaries. After abstract and title screening, ten studies were assessed for eligibility via full text, of which five studies met our pre-specified inclusion criteria for analysis (Figure 1).
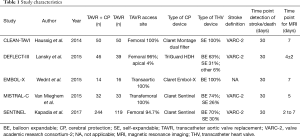
Study characteristics of the included studies can be found in Table 1. A total of 643 patients were included in the present analysis, of which 386 patients were randomized to TAVR + CP and 257 with TAVR only. Assessment for risk of bias can be found in Table S1.
Full table

Full table
Baseline patient characteristics
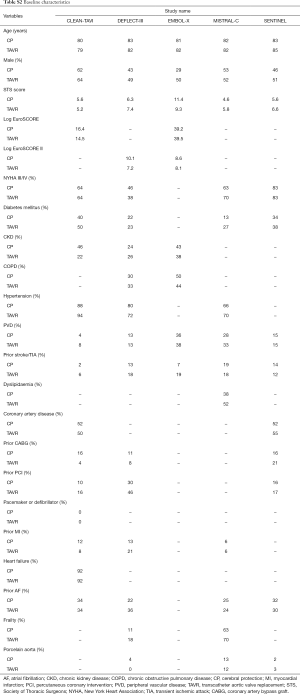
The baseline patient characteristics and meta-analyses comparing patients randomized to TAVR + CP versus TAVR alone can be found in Table S2. No significant difference in age was found amongst patients randomized to TAVR + CP versus TAVR alone (weighted mean difference 0.26; 95% CI, −1.10 to 1.63) (Figure S1). There were 48% males in the TAVR + CP group compared to 53% males in the TAVR only group, which was not statistically significant (OR, 0.83; 95% CI, 0.60 to 1.15). The operative risk between both groups was also similar, with no significant difference in Society of Thoracic Surgeons (STS) score (weighted mean difference, 0.21; 95% CI, −0.81 to 1.23), Log EuroSCORE (weighted mean difference, 1.56; 95% CI, −1.82 to 4.93) and Log EuroSCORE II (weighted mean difference, 1.65; 95% CI, −0.83 to 4.12). There were no significant differences in other patient characteristics at baseline. No significant heterogeneity existed between studies for all baseline variables (I2=0%). Inspection of funnel plots revealed no evidence of publication bias (Figure S2).
Full table

Clinical outcomes
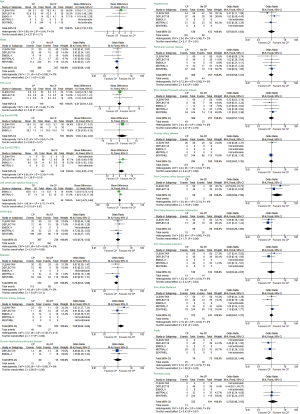
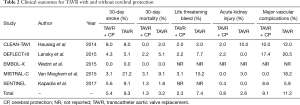
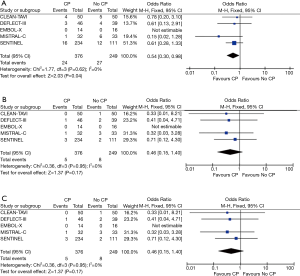
The major clinical event rates are shown in Table 2. Overall 30-day stroke rates were 5.4% in the TAVR + CP group compared to 9.3% in the TAVR group. Overall 30-day mortality rates were 1.3% in the TAVR + CP group compared to 3.2% in the TAVR group. There was a significant reduction in the primary composite outcome of death/stroke in patients randomized to TAVR + CP compared to TAVR alone (OR, 0.54; 95% CI, 0.30 to 0.98) (Figure 2A). There was no significant reduction in the rate of stroke in patients undergoing TAVR + CP versus TAVR alone (OR, 0.46; 95% CI, 0.15 to 1.40) (Figure 2B). Mortality rate was lower in patients randomized to TAVR + CP compared to TAVR alone, although the result was not significant (OR, 0.46; 95% CI, 0.15 to 1.40) (Figure 2C). Rates of life threatening bleed were also lower in patients undergoing TAVR + CP compared to TAVR, although the result was not significant (OR, 0.30; 95% CI, 0.08 to 1.14) (Figure 3A). There were also no significant differences in rates of acute kidney injury (OR, 0.49; 95% CI, 0.15 to 1.67) (Figure 3B) and major vascular complications (OR, 0.30; 95% CI, 0.08 to 1.14) (Figure 3C). There was no significant heterogeneity between studies for all clinical outcomes (I2=0%). Inspection of funnel plots revealed no evidence of publication bias (Figure S3).
Full table

Imaging outcomes
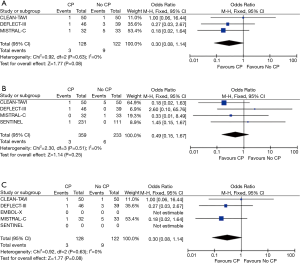
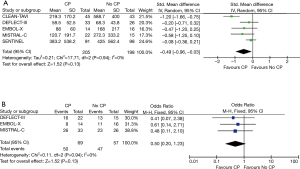
In all five studies, MRIs were performed between days 2 to 7 for assessment of total brain lesions. There was a significant reduction in total lesion volume in patients undergoing TAVR + CP versus those patients without TAVR + CP (standardized mean difference, −0.49; 95% CI, −0.96 to −0.03) (Figure 4A). There was significant heterogeneity between studies for total lesion volume (I2=77%). TAVR + CP was associated with a 50% reduction in patients with new brain lesions, although the result was non-significant (OR, 0.50; 95% CI, 0.20 to 1.23) (Figure 4B). There was no significant heterogeneity between studies reporting number of patients with new brain lesions (I2=0%). Inspection of funnel plots revealed no evidence of publication bias (Figure S4).

Discussion
The clinical impact of embolized debris into the brain and the long-term sequelae of silent cerebral lesions detected by DW-MRI remains a topic of controversy. Although there are studies which have associated the presence of such silent infarcts to neurocognitive decline (15,16), this evidence remains limited and thus such findings are only a surrogate marker for neurological events. However, given the lack of significant benefit of CP devices with regards to hard clinical endpoints including strokes and death, it is difficult to make any strong guidelines or recommendations regarding its use. Limited sample sizes and inadequate statistical power have limited interpretation of clinical endpoints to date. In light of this, we attempted pool the current evidence to provide a more robust and powered analysis of the clinically relevant endpoints. In this systematic review and meta-analysis, we found that TAVR + CP was associated with a reduction in the composite endpoint of death or stroke when compared to TAVR alone. These findings suggest that TAVR + CP may be a suitable treatment strategy for patients with severe aortic stenosis and warrants further trials with larger sample sizes.
The PARTNER II cohort A trial reported 30-day stroke rates of 5.5% after TAVR, which confirmed the significant risk of cerebrovascular events in intermediate risk patients (4). This has led to the development of CP devices, most commonly filters placed in the brachiocephalic and common carotid arteries that capture embolic debris dislodged during TAVR. Based on the small number of studies available, CP devices capture debris in nearly all patients undergoing TAVR (12,13,17). In the SENTINEL trial, 99% of patients had captured debris, which included frequent thrombus, artery wall, valve tissue and calcification. Theoretically, a device that captures debris should reduce cerebrovascular events, yet data from available studies have failed to demonstrate improvements in hard clinical outcomes. The present study demonstrates that there may be a role for CP devices as a clinically benefit adjunct to TAVR procedures.
There may be several reasons why the individual RCTs have not demonstrated significant improvements in hard clinical outcomes (11-14,18). The available studies are likely underpowered to identify significant improvements. The largest trial to date, the SENTINEL trial, showed a 38% reduction in strokes at 30 days, that was non-significant (12). However after pooling trials from the five RCTs, the data pool included a total of 386 patients randomized to TAVR + CP and 257 patients to TAVR only. The meta-analysis suggests that CP devices are associated with a 46% reduction in stroke/death.
The use of brain lesion volume on MRI scans as a marker of cerebrovascular disease has led to mixed results in the available RCTs (11-13). The CLEAN-TAVI trial and the MISTRAL-C trial showed significant reductions in new lesion volume, whilst the SENTINEL trial showed a 42% reduction in new lesion volume that was non-significant. In the SENTINEL trial, post hoc analyses revealed significant reductions after adjustment for baseline brain lesions on MRIs. Furthermore, the authors used an MRI window of 2 to 7 days which may have been too broad, resulting in significant heterogeneity in lesion volume (12). Our pooled meta-analysis demonstrated a significant reduction in damaged brain volume. The significant heterogeneity in the meta-analysis emphasises the importance of standardization of DW-MRI scans, time points for evaluation of brain lesions, standardization of multiple TAVR devices and implantation techniques.
Subclinical ischemic brain injury has been linked to cognitive and functional neurological impairment over time (12,15,19-21). The prevention of subclinical embolization may continue to be an important marker of cerebrovascular outcomes, particularly when treating lower risk patients with severe aortic stenosis. The source of these new silent cerebral lesions remain unclear—whether it is due to the CP devices’ incomplete capture of emboli or whether it is iatrogenically produced from manipulation and positioning of the CP devices in the aortic arch and great vessels (22)—remains to be determined. The suggested benefit from the present analysis warrants further research into CP devices, where preventing procedure-related cerebral injury may have important long-term sequelae.
The safety of CP devices in TAVR has been validated in RCTs comparing TAVR + CP and TAVR, with similar in-hospital and 30-day composite safety endpoints (11,12,18). We showed a significant reduction in rates of major bleeding and acute kidney injury in patients undergoing TAVR + CP compared to CAVR and a non-significant reduction compared to TAVR alone. Despite the additional manipulation and positioning of the CP devices, there was no difference in rates of vascular complications between TAVR + CP and TAVR. TAVR is associated with a small increase (15–18 minutes) in procedure time (11,12) in order to obtain arterial access, device positioning, filter deployment and filter recapture and device removal.
Patients undergoing TAVR are often older, frail, and affected by multiple comorbidities, implying a significant risk for thromboembolic cerebrovascular events (1,23). It is plausible that the benefit may be maximal in patients at high risk of cerebrovascular events and the importance of careful patient selection for CP devices may become an integral part of the clinical decision making.
Limitations
The present study has several limitations. These include inherent limitations included in the individual RCTs: study design, sample size, treatment crossover and patient drop out. Secondly, there is currently a lack of standardized reporting practices with regards to DW-MRI measures, including the mixed use of 3T versus 1.5T scanners, and a broad window for interpretation of 2–7 days for some studies. Despite this variation in the techniques used in the assessment of lesion volume, there was no significant heterogeneity in the assessment of clinical outcomes found in the present meta-analysis. Standardization of brain lesion volume assessment via MRI is warranted. Future trials of CP devices should follow the recent guidelines for the assessment of neurological endpoints in cardiovascular trials (24). Differences in valve types may also have contributed to some heterogeneity. In the SENTINEL trial, SAPIEN 3 was associated with lower new brain lesions compared with Evolut R or SAPIEN XT, which limited the benefit that patients on the SAPIEN 3 could derive. In addition a trend towards higher number of patients without any new lesions was seen for the SAPIEN 3 valve in DEFLECTIII. A number of CP devices were also employed in this meta-analysis, including Claret, EMBOL-X and Triguard, it is currently still unclear whether the outcomes of these three devices are equivalent or not. Differences in the vascular access, deployment process and method of embolic protective modality between the three devices may also contribute to some heterogeneity.
Conclusions
The current evidence suggests that TAVR + CP is safe and may be associated with significant reductions in death/stroke compared to TAVR alone. The lack of benefit in hard clinical outcomes from the individual trials, warrant large adequately powered RCTs with standardised assessment of endpoints.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010;121:870-8. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Wang N, Lal S. Post-dilation in transcatheter aortic valve replacement: A systematic review and meta-analysis. J Interv Cardiol 2017;30:204-11. [Crossref] [PubMed]
- Kahlert P, Al-Rashid F, Döttger P, et al. Response to letters regarding article, “cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study Circulation 2013;127:e591-2. [Crossref] [PubMed]
- Nombela-Franco L, Webb JG, de Jaegere PP, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 2012;126:3041-53. [Crossref] [PubMed]
- Astarci P, Glineur D, Kefer J, et al. Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and trans-apical approaches using Edwards Sapiens valve. Eur J Cardiothorac Surg 2011;40:475-9. [PubMed]
- Fairbairn TA, Mather AN, Bijsterveld P, et al. Diffusion-weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart 2012;98:18-23. [Crossref] [PubMed]
- Ghanem A, Müller A, Nähle CP, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol 2010;55:1427-32. [Crossref] [PubMed]
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a Cerebral Protection Device on Brain Lesions Following Transcatheter Aortic Valve Implantation in Patients With Severe Aortic Stenosis: The CLEAN-TAVI Randomized Clinical Trial. JAMA 2016;316:592-601. [Crossref] [PubMed]
- Kapadia SR, Kodali S, Makkar R, et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2017;69:367-77. [Crossref] [PubMed]
- Van Mieghem NM, van Gils L, Ahmad H, et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: the randomised MISTRAL-C trial. EuroIntervention 2016;12:499-507. [Crossref] [PubMed]
- Wendt D, Kleinbongard P, Knipp S, et al. Intraaortic Protection From Embolization in Patients Undergoing Transaortic Transcatheter Aortic Valve Implantation. Ann Thorac Surg 2015;100:686-91. [Crossref] [PubMed]
- Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215-22. [Crossref] [PubMed]
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064-89. [Crossref] [PubMed]
- Schmidt T, Schlüter M, Alessandrini H, et al. Histology of debris captured by a cerebral protection system during transcatheter valve-in-valve implantation. Heart 2016;102:1573-80. [Crossref] [PubMed]
- Lansky AJ, Schofer J, Tchetche D, et al. A prospective randomized evaluation of the TriGuard HDH embolic DEFLECTion device during transcatheter aortic valve implantation: results from the DEFLECT III trial. Eur Heart J 2015;36:2070-8. [Crossref] [PubMed]
- Floyd TF, Giovannetti T. Neurocognitive outcomes in older adults after transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2012;144:1539. [Crossref] [PubMed]
- Svensson LG, Blackstone EH, Apperson-Hansen C, et al. Implications from neurologic assessment of brain protection for total arch replacement from a randomized trial. J Thorac Cardiovasc Surg 2015;150:1140-7.e11. [Crossref] [PubMed]
- Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611-9. [Crossref] [PubMed]
- Rodés-Cabau J, Kahlert P, Neumann FJ, et al. Feasibility and exploratory efficacy evaluation of the Embrella Embolic Deflector system for the prevention of cerebral emboli in patients undergoing transcatheter aortic valve replacement: the PROTAVI-C pilot study. JACC Cardiovasc Interv 2014;7:1146-55. [Crossref] [PubMed]
- Giustino G, Dangas GD. Stroke prevention in valvular heart disease: from the procedure to long-term management. EuroIntervention 2015;11 Suppl W:W26-31.
- Lansky AJ, Messé SR, Brickman AM, et al. Proposed Standardized Neurological Endpoints for Cardiovascular Clinical Trials: An Academic Research Consortium Initiative. J Am Coll Cardiol 2017;69:679-91. [Crossref] [PubMed]


